Backing off Prostate and Cervical Cancer Screening

Over-diagnosis- Diagnosis of a disease when no symptoms are present.
Over-treatment- Treatment of a disease when it is not life-threatening.
A major area of cancer research being examined these days is screening methods. Specifically the question is how often should these tests be run and on what population groups for a given type of cancer. There is now enough historical data to reach a consensus going forward. Bearing in mind the purpose of cancer screening is to detect cancer at an early enough stage when it is treatable. If a given frequency of screening is not preventing death from that particular cancer then the guidelines can be augmented. With every medical procedure, diagnostic tests included, there are downsides. The major downside to these types of tests is the over-diagnosis and over-treatment of the disease. The problem with this type of screening is if something is detected it opens the door on whether to treat the could-be cancer or not. Not to mention there is always the possibility of a false-positive test result. Cancer treatment methods are harsh- the risk to benefit ratio is so skewed by the toll cancer can take on one’s body. This allows cancer drugs themselves to reap a pretty hefty toll on the individual all in the name of stopping the cancer. It is because of this that researchers are now taking a very serious look at whether or not guidelines on cancer screening are appropriate- how often to test and which population to test.
Cancer screening methods that quickly come to mind are the Prostate Specific Antigen (PSA) test for Prostate Cancer and the cervical smear, better known as a Pap smear, for detection of early Cervical Cancer. The Prostate Cancer test determines a man’s level of PSA in his blood. While the Pap smear is a cytological examination looking for abnormalities in cell growth in cervical cells, based on collection of a tissue sample. These tests are done to try and catch cancer early when it is more likely to respond to treatment. Since the inception of these early detection methods there is enough research to allow for adjustments to recommendations of using these tests. Recently the utilization of both the PSA test for men and the cervical smear for women have been scaled back.
Prostate Cancer is the second leading cause of cancer deaths in men in the US based on statistics from 2012- it caused over 28,000 deaths that year.
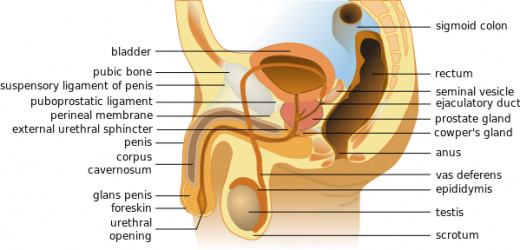
The prostate gland produces a component of seminal fluid that aids sperm in fertilization. This gland is located below a man’s bladder and in front of his rectum. It surrounds the urethra, which is the urinary tract tubing. A healthy prostate is the size of walnut while an unhealthy one can grow larger and squeeze the urethra, leading to several painful urinary tract issues. An enlarged prostate can be caused by either a benign tumor-Benign Prostatic Hyperplasia (BPH)-or a malignant tumor-Prostate Cancer (PC). PSA is an enzyme, specifically a serine protease, and is made almost exclusively by the prostate gland via epithelial cells. The amount of PSA can be detected by a blood test, where levels elevated over 4.0 nanograms/milliliter are cause for concern. A given man’s PSA level is taken into account with his data from previous PSA testing, his age, and family history of Prostate Cancer. The digital rectal exam, DRE, is the notorious physical examination of a man’s prostate that is often used in conjunction with the PSA exam.
When a PSA test is considered positive-indication of early detected prostate cancer- the following options present themselves. The PSA test can be redone and then used as a monitoring tool to watch for an additional increase in the levels, this is a conservative option. Other tests can be done to rule out another reason for the increase in PSA such as a urine test to detect a urinary tract infection. Diagnostic imaging can be done to gain more information as well. A non-conservative option is to biopsy the prostate to determine if the cells present are cancer cells. From the biopsy about 25% of men turn out to actually have cancer, which means the majority had an elevated level of PSA for a reason other than cancer or they are within the false-positive rate of the screening method. In the case of confirmed Prostate Cancer, a radical prostatectomy can be done, radiotherapy, chemotherapy, hormone therapy, or active surveillance, in which the patient would be closely monitoring using the above diagnostic tests to establish if the cancer is getting worse, are all possible treatment options. Complications from these various treatments include disruption to both normal sexually health (erectile dysfunction) and normal continence, either bowel or urinary or both, as well as infection to the area.
Prostate Cancer Stages
Stage I
| Cancer is contained to prostate gland
|
Stage II
| Still contained to gland but more advanced
|
Stage III
| Cancer spreads to local areas such as the seminal vesicles
|
Stage IV
| Spread to bladder, rectum, and other nearby structures. Also may spread to lymph nodes, bones, etc.
|
The U.S. Preventative Services Task Force (USPSTF) used several studies to base their recommendations for the discontinuation of PSA screening in all men in the US as of May 2012. The National Cancer Institute (NCI) conducted the PLCO (Prostate, Lung, Colorectal, and Ovarian) study in the US from 1993 to 2001 that asked the question, separately for each of these cancers, does screening save lives? For prostate cancer, men (aged 55-74) in the interventional arm received six annual PSA tests (and four annual DREs) over the years while the control group received usual care from their own doctors which may or may not have included prostate screening. The trial saw no statistical difference in terms of death from Prostate Cancer between the two groups. The control group did have a higher diagnosis of PC but there was not an increase in death from Prostate Cancer seen in the usual care group. The take home from this study is the additional screening in the interventional arm didn’t prevent death from PC. Another study, the ERSPC (European Randomized Study of Screening for Prostate Cancer) did see a decrease in death from PC in the intervention arm (which in this study was PSA testing every two to four years). This study reported a 29% decrease in the risk of dying from PC with screening.
The major difference in the US study compared to the European study was that many of the US participants had already received a PSA test before being randomized to either the control or the interventional arm. In the European study most of the men had not had a previous PSA test before joining the study. The USPSTF made their screening guidelines decision weighing both studies and, of course, with their decision comes criticism. One of the directors of the ERSPC trial, Chris Bangma, points out the contamination of the sample of US men needs to be accounted for in the results; removal of all subjects who had already had a PSA test before the study greatly reduced the statistical power achieved and thus the influence that trial imparts. Also Carlsson et al. point out the USPSTF made their recommendation before the ERSPC trial had fully concluded and that it was the follow-up data that gave the indication that the screening did in fact prevent PC-caused death. It is important to note that researchers and physicians alike are hard at work to come up with more accurate Prostate Cancer detection methods.
The PSA-Prostate Cancer screening program isn’t the only one that underwent scrutiny this past year. Cervical Cancer screening has also been subject to a re-evaluation now that there is enough historical data to allow for it. Almost every single case of Cervical Cancer is caused by Human Papillomavirus (HPV), a virus that about half of all sexually active people- men and women- will be infected with at one point in their adult lives. HPV is sexually transmitted and once present can cause genital warts or various forms of cancer in women and men. There are more than 150 different varieties of HPV where the ones causing genital warts are known as low-risk types and the ones causing cancer are high risk. Most HPV infections go away on their own after a year or two and generally are unknown to the individual. The longer the infection stays the more likely it will lead to cancer; the virus can mutate overtime and go undetected by the immune system.
Statistics from 2011 put Cervical Cancer as the second most common cancer in women worldwide.
The cervix is the lower narrow portion of a women’s uterus, it connects the vagina with the uterus. It is part of the route sperm take to get to the egg and the cervix functions in producing mucus to aid the sperm on their journey. The Papanicolaou smear or test, more commonly abbreviated to the Pap smear or test, is a test named for the doctor who invented it. It involves taking a sample of cervical tissue and examining the cells under the microscope for abnormalities indicative of precancerous states or cancerous states. An HPV DNA test, known as the HPV High-Risk DNA Hybrid Capture II can be done on the similar cellular sample to identify the most notorious HPV strains, 16 and 18, as well as eleven others known to cause cancer. The HPV diagnostic test is currently only approved as a follow-up to an abnormal Pap smear of the cervix. There is no approved test for HPV infection in men and no approved HPV test for the other types of cancers caused by HPV. HPV vaccination is recommended for young women, before they become sexually active, in addition to regular cervix screening when age-appropriate.
Precancerous Staging in Cervix -Noninvasive Squamous Lesion
CIN I
| Mild dysplasia
|
CIN II
| Moderate dysplasia
|
CIN III
| Severe dysplasia-Equal to Cervical Cancer Stage Zero
|
Dysplasia is abnormal cell growth which can mean unusual shaped cells, more dividing cells that normal for a given tissue, or more immature cells in an area where generally mature cells are present.
When Cervical Cancer is detected by the Pap/HPV test combination, colposcopy-a form of microscopy that allows for visualization of the cervix and surrounding areas- will be done and usually a biopsy is taken at the time of this procedure. There are several methods that can be done to take the biopsy sample but unfortunately all sound invasive and painful. The precancerous staging scheme (on the right) is the ranking assigned to the presence of abnormal growth detected via the colposcopy and/or biopsy. The final stage CIN III corresponds to stage zero of Cervical Cancer, the switch over from precancerous to cancerous in the case of the cervix is when the cells travel outside of the cervical epithelium into other parts of the cervix. As one can imagine adverse effects of any of these colposcopy/biopsy methods is bleeding and/or cramping. In the case of confirmed cancer, the stage dictates the treatment course. If the cancer is detected early, in stage I or II, surgery can be done. Different surgical options are available depending on how far the cancer has spread. Radiotherapy is recommended for any stage on its own or in conjunction with chemotherapy and can also be done before or after any of the mentioned surgeries. Surgery is not an option as the cancer progresses and chemotherapy is not done on its own for curative purposes, instead it is used to amplify the effects of radiotherapy or can be used to contain late stage Cervical Cancer. In addition to the staging of the cancer, whether the woman hopes to get pregnant in the future also weighs on the treatment plan.
Cervical Cancer Staging
Stage I
| Cancer only in cervix
|
Stage II
| Spread to upper part of vagina, maybe lower parts as well and/or other nearby tissue
|
Stage III
| Spread to pelvic wall, lower part of vagina. May have kidney dysfunction due to blockage based on tumor size
|
Stage IV
| Spread to bladder or rectum or may travel to distant organs/areas.
|
New Pap Smear Guidelines
Age
| Frequency of Pap Smear
|
|---|---|
21 to 29
| Every Three Years
|
30 to 65
| Every Five Years
|
65+
| No test (normal history)
|
The guidelines surrounding the recommended frequency of Pap smear testing has recently been modified in 2012. Data on this has been accumulating since the test’s inception at annual physicals for women beginning in the 1940s. Recently it has been thoroughly looked into if having the test done annually is necessary. Research has been carried out and the bigwigs- The American Cancer Society (ACS), the American College of Obstetricians and Gynecologists (ACOG), and the aforementioned USPSTF, - agree it is not necessary for a woman of any age to have an annual pap test done. The ACOG cites their reasoning for this decrease in testing since most cervical cancer cases are detected in women who were not being screened on a regular basis. One unintended effect of this reduction in Pap test recommendation is will this lead to women not going to their annual physical? Personally I think this reduction in the guidelines will decrease women’s well visits to their primary care doctors, resulting in a lapse of monitoring other important health issues.
Evidence Based Medicine combines many resources on a subject in order to determine how best to treat a patient, this involves using both clinical expertise and data from research.
These cancer screening methods illustrates a perfect example of Evidence-Based Medicine. These tests were designed decades ago to detect early cancer and doctors made the call at their inauguration to air on the side of caution and recommend yearly screening for these tests in conjunction with one’s annual physical. This testing schedule wasn’t based on evidence since there was no evidence out there yet about how to proceed with these screening tactics. But now there is data from previous years for many medical procedures hence this new-found terminology and practice of Evidence-Based Medicine. Physicians and medical groups can review the data on cancer screening and agree on a reasonable schedule that is safe for the patient while at the same time does not unnecessarily cause stress to the patient, as well as drain resources and time of the health care community. Ultimately an individual’s history, age, and health record will continue to serve as the main things taken into consideration by a doctor as to the person’s cancer screening schedule. But these guidelines will certainly be taken as rule by health professionals and insurance companies alike for the general population, which may lead to difficulties obtaining a test at the insistence of an individual patient if the scenario falls outside of the guidelines.
Sources available upon request.