Genetic Alterations in Non Small Cell Lung Cancer
Introduction
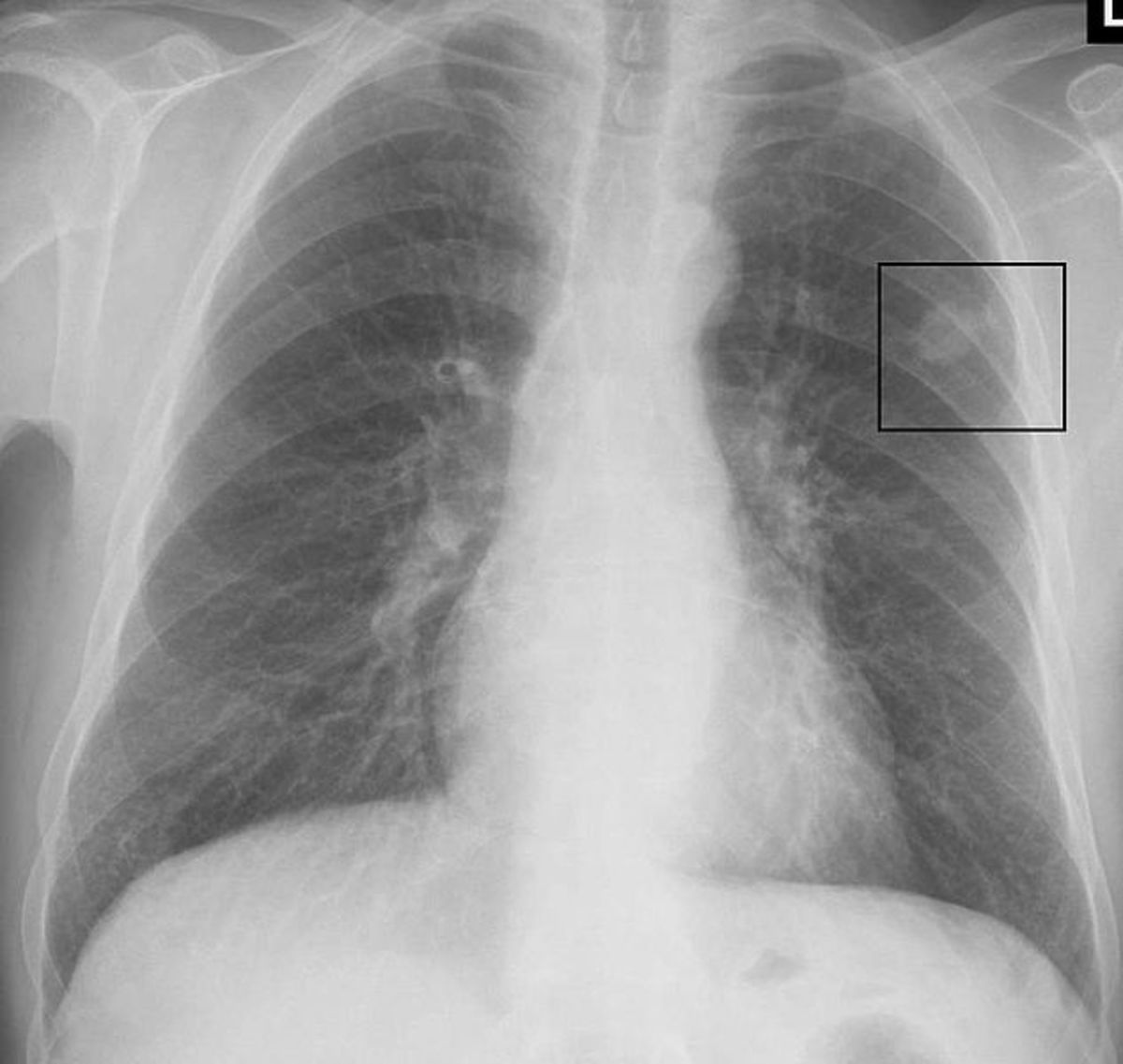
There are two main classifications of primary lung cancers, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) of which, Non small cell makes up around 75% of lung cancer deaths and is classified further as squamous cell carcinoma (SCC), adenocarcinoma or large cell carcinoma (LCC) and it will be the focus of this review.
NSCLC tumors have similar genetic alteration patterns as those that arise in other anatomical areas i.e. a step wise accumulation of genetic events that result in the development from benign (non cancerous) bronchial epithelial tissue into neoplastic (cancerous) tissue.
Most solid tumors are unstable at two different levels, chromosomal instability and micro-satellite instability. The genetic alterations that occur within SCLC and NSCLC tumors occur within the genes that underlie the growth stimulating signalling pathways, the tumor suppressor pathways and to a lesser extent those within the cell immortilisation and the avoidance of cell death pathways. Some forms of NSCLC do arise from chromosomal rearrangements such as 8q gain in some squamous cell cancers and adenocarcinoma’s as studied by Sy et al (2004).
Stop Smoking Information
Cigarette Smoke and NSCLC
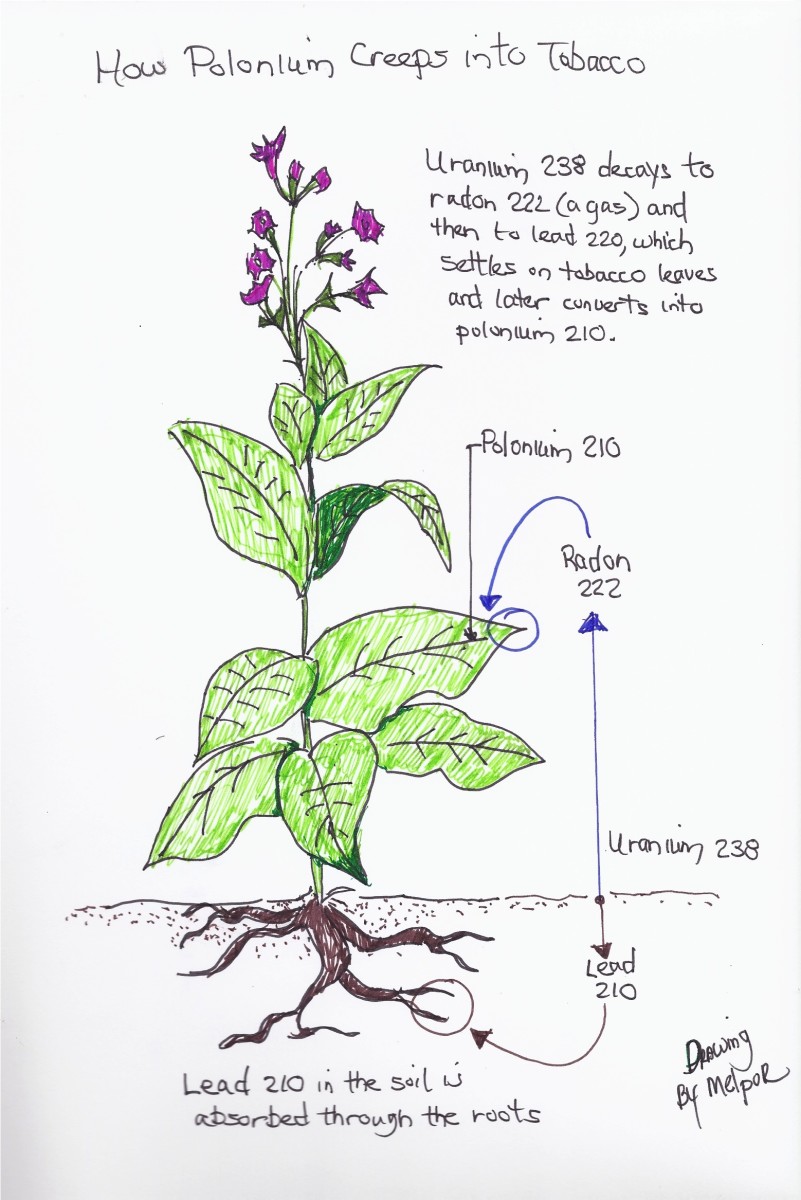
The cause of the majority of these genetic changes are clearly known and the statistical evidence strongly suggests that cigarette smoke plays a heavy role in lung tumor development particularly the SCC sub type of NSCLC. The rise in cigarette smoking over the last century has closely paralleled the rise in lung cancer. In 1941 lung cancer accounted for about 5000 deaths in England and Wales and rose to 35 000 in 1984 (Little et al, 2007). A study by Little et al (2007) found that 93% of their patients suffering from NSCLC were smokers, statistically implicating smoking in the development of cancer. Cigarette smoke contains carcinogens such as benzene and ethylene oxide, these cause progressive changes in the bronchial mucosa and carcinoma is preceded by squamous metaplasia and dysplasia. These cells are seen far more commonly in the sputum of smokers, than sputum from non-smokers with the number of cigarettes smoked daily proportional to the increased shed of abnormal cells.
Although there has been a significant decrease in smoking rates (specific areas of the world) mortality from the disease still remains high specifically in men, though ex-smokers do carry a significantly increased risk for many years after quitting, with adenocarcinoma being higher in ex-smokers and non-smokers than SCC. Nicotine itself is not carcinogenic but is however the reason why people keep smoking. Tobacco smoke contains small amounts of polycyclic aromatic hydrocarbons (PAH and 4-(methylnitrosamino)-1(3-pyridyl)-1-butanone (NNK) and these require metabolic activation to excerpt the carcinogenic effects (Zandwijk, 2001). The metabolic activation leads to the formation of DNA adducts which are when DNA is bound covalently to the carcinogenic metabolites. If these abducts escape the DNA repair mechanisms, including apoptosis, they may induce miscoding which results in a permanent mutation and tumour development (Zandwijk, 2001).
Different Genetic Alterations in NSCLC
The arm of chromosome 3p holds the most frequent and earliest genetic alterations causing lung cancer development (100% in SCLC and ≥ 90% in NSCLC.) These may have an effect on more than one tumour suppressor gene located within that arm and their role in NSCLC development has been reviewed by Sato et al (2007). RASSF1A gene is involved in multiple pathways which are critical to cancer pathogenesis. It is rarely mutated in lung cancer however its expression is lost due to tumour acquired promoter methylation in approximately 90% of small cell and approximately 50% of non small cell lung tumours (Sato et al (2007)). Located directly adjacent to the RASSF1A gene is the tumour suppressor gene FUS1. This gene also rarely mutates in lung cancer. Its messenger RNA is usually expressed but its expression is lost in lung tumours, however its wild type form is expressed in normal lung cells. In its wild type form FUS1 induces G1 growth arrest and apoptosis. Loss of expression of FUS1 therefore, causes failure of cell regulatory mechanisms and neoplastic growth.
Transforming growth factor beta is a multi functional cytokine that plays a paradoxal role within lung cancer. This cytokine plays many functional roles within cell survival, proliferation and immunosurveliance (Sato et al, 2007). In terms of lung cancer TGF-β inhibits cell proliferation and initiates apoptosis in both SCLC and NSCLC. However at a later stage of tumour development TGF-β is responsible for initiating angiogenesis and at this point the inhibitory effects of the protein are lost by differing mechanisms. In NSCLC, mutations within TGF- are not frequent however mutations in mechanisms upstream are more frequent and lead to inhibition of TGF-β (Sato et al, 2007).
Epigenetic alterations have significant roles to play in cancer development particularly DNA methylation, (addition of a methyl group to cytosine’s proceeding guanidines). If this occurs in the promoter regions of tumour suppressor genes it can lead to gene silencing or inactivation leading to tumour development. A study by Hawes et al (2009) examined DNA methylation of selected genes to quantify if these play a role in the development of NSCLC. They examined 117 patients with NSCLC and found that methylation of a number of genes was associated with histological subtype.
References
Cancer Research UK. (2009, June 17). Lung cancer and smoking statistics - Key Facts. Retrieved November 15, 2009, from Cancer Research UK: http://info.cancerresearchuk.org/cancerstats/types/lung/index.htm?script=true
Esposito, V., Baldi, A., De Luca, A., Micheli, P., Mazzarella, G., Baldi, F., et al. (1997). Prognostic Value of p53 in Non-Small Cell Lung Cancer: Relationship with Proliferating Cell Nuclear Antigen and Cigarette Smoking. Human Pathology 28 , 233-237.
Hawes, S., Stern, J., Feng, Q., Wiens, L., Rasey, J., Lu, H., et al. (2009). DNA hypermethylation of tumours from non-small cell lung cancer (NSCLC) patients is associated with gender and histologic type. Lung Cancer .
Little, A. G., Gay, E., Gaspar, L., & Stewart, A. (2007). National survey of non-small cell lung cancer in the United States:Epidemiology, pathology and patterns of care. Lung Cancer 57 , 253-260.
Puisieux, A., Lim, S., Groopman, J., & Ozturk, M. (1991). Selective Targeting of p53 Gene Mutational Hotspots in Human Cancers by Etiologically Defined Carcinogens. CANCER RESEARCH 51 , 6185-6189.
Sato, M., Shames, D., Gazdar, A., & Minna, J. (2007). A Translational View of the Molecular Pathogenesis of Lung Cancer. Journal of Thoracic Oncology. 2 , 327-343.
Sy, S. -H., Wong, N., Lee, T. -W., Tse, G., Mok, T., Fan, B., et al. (2004). Distinct patterns of genetic alterations in adenocarcinoma and squamous cell carcinoma of the lung. European Journal of Cancer. 40 , 1082-1094.
Tamboli, P., & Ro, J. (2002). Lung Cancer: Chapter 4; Pathologic Evaluation of Lung Cancer. New York: Springer-Verlag New York, Incorporated .
Tammemagi, M. C., Mclaughlin, J. R., Mullen, B. M., Bull, S. B., Johnston, M. R., Tsao, M.-S., et al. (2000). A Study of Smoking p53 Tumour Supressor Gene Alterations and Non-Small Cell Lung Cancer. Annals Of Epidemiology. 10 , 176-185.
Zandwijk, N. v. (2001). Chemoprevention of lung cancer. Lung Cancer. 34 , s91-s94.