Is There a Cure for Cancer and Regeneration of Damaged Tissues?
Stem Cells
Overview
The issue of stem cell treatment is a complex and unfamiliar topic for most people. After researching this topic and interviewing medical professionals, we visited Dr. Shimon Slavin in Israel. What we learned was both fascinating and frustrating. Why are these treatments prevented in the US? Why are people being medicated instead of cured?
Because this topic of stem cells may not be familiar to everyone, I have included a glossary at the end of the article.
The entire document was co-authored and approved by Dr. Slavin.
Stem Cell Therapy--A Miracle Within
On a recent visit to Tel Aviv, Israel, we interviewed a well-known doctor in the field of Stem Cell Therapy, Dr. Shimon Slavin, professor of Medicine, and the Medical and Scientific director of the International Center for Cell Therapy and Cancer Immunotherapy (CTCI) in Tel Aviv. His research has been well documented and he travels around the world speaking to doctors and researchers in this amazing and untapped area of medicine. Our initial purpose of the interview was to discuss the way America lags behind in clinical application of stem cell research, primarily because of the media hype about unethical embryonic stem cell research, which is not what he is doing to start with, and not what anyone should be doing at this point in time. That is a topic for another day.
What we learned is that stem cell therapy seems to be the most promising future approach for treatment of many malignant and non-malignant diseases caused by acquired or congenital conditions, including 1 senescence of organs due to the aging process, and for clinical conditions currently considered incurable.
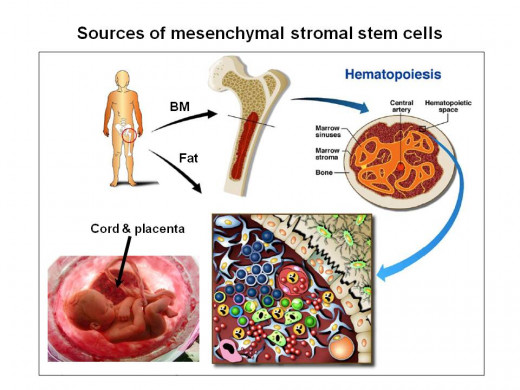
In general, there are two types of stem cells used for treatment at CTCI; 2 Hematopoietic stem cells which can generate all the circulating blood cells (red blood cells, white blood cells, platelets and the entire immune system); and 3 Mesenchymal stromal cells (MSC) which can regenerate cells belonging to different tissues by direct differentiation and/or by secretion of factors that can help minimize the damage caused by disease or aging.
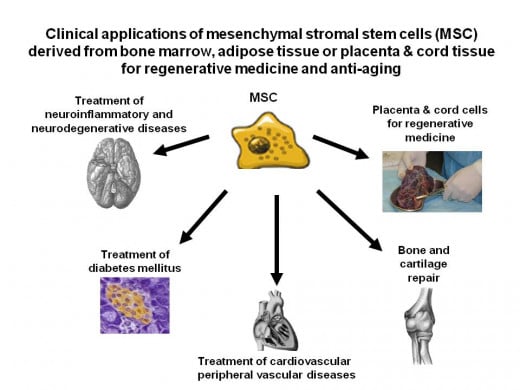
Treatment with stem cells for malignant diseases, as well as a long list of genetic diseases, immune deficiency and deficiency of bone marrow products and certain genetic diseases are very successful. These are treatable by allogeneic stem cell transplantation and can be accomplished using a patient’s own stem cells collected from the bone marrow or the blood, following mobilization from the bone marrow by growth factors. The other type of multi-potent cells that be used for regenerative medicine can be also isolated and enriched from the bone marrow, 4 adipose tissue, or from 5 cryopreserved placenta and cord tissue. Placenta and cord tissue cells can be best derived from a normal baby related to the recipient, but it seems that readily available, “off-the-shelf” placenta and cord tissue derived MSCs may be also useful for treatment of any unrelated patients in need for regenerative/anti-aging indications.
There are many different diseases that can be successfully treated by the first type of stem cells obtained from the bone marrow or collected from the blood. Such stem cells are approved for the use for treatment of different diseases all over the world. For instance, some types of cancer, including acute and chronic leukemia, Hodgkin’s and Non-Hodgkin’s lymphoma, multiple myeloma, myelodysplastic syndromes and some other malignancies incurable by conventional chemotherapy. Using stem cell transplantation, cure can be accomplished with bone marrow derived stem cells, even for patients considered incurable by conventional procedures. Stem cell transplantation with donor-derived stem cells based on fully matched, or partially mismatched family member, or matched unrelated donor seems to be more effective for complete cure of patients with malignant and non-malignant diseases as compared with 6 autologous stem cells because the immune system of the donor that replaces patient’s own immune system can eliminate malignant cells with a mechanism that resembles “reverse rejection”, the so called cell-mediated anti-cancer immunotherapy, resulting in induction of graft-versus-leukemia (GVL) or graft-vs.-tumor (GVT) effects.
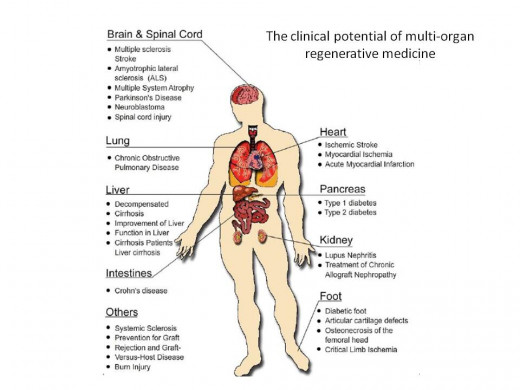
At Slavin’s center at CTCI they are now using new approaches for treatment of cancer based on cell therapy without using stem cells. Such methods have been successful at curing many types of cancers since 1986 using a combination of approaches, focusing on 7 immunotherapy by activated killer cells of intentionally mismatched donors, and special anti-cancer vaccines. Targeting killer cells against residual malignant cells can be accomplished using 8 monoclonal and bi-specific antibodies against determinants expressed on the surface of cancer cells. In parallel, CTCI provides experimental regenerative medicine treatment for a broad spectrum of non-malignant indications such as neuroinflammatory and neurodegenerative disorders (for example: multiple sclerosis; amyotrophic lateral sclerosis; Parkinson’s disease; stroke); chronic ischemic heart disease; renal insufficiency, and for regeneration of cartilage in the knee joint, and soon for treatment of diabetes mellitus, all based on the unique multi-potent capacity of MSCs. MSCs can both differentiate into cells resembling nearly every tissue as well as secrete growth factors that can help regenerate and replace damaged cells.
We don't allow similar treatments in the USA. Why? “In my humble opinion” says Slavin, “it is because of a combination of insurmountable hurdles of regulatory authorities on the one hand, and lack of support by pharmaceutical agencies control what is allowed and what is prohibited based on financial interests. They have immense influence on the FDA, which must approve all types of treatments and they are not always flexible either. Too much concern about safety comes at the expense of delaying potentially effective anti-cancer treatment and cell-mediated procedures for regenerative medicine for so many patients in need.”
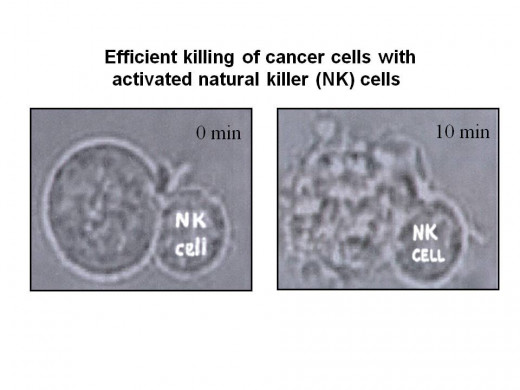
Anti-cancer immunotherapy says Dr. Slavin is based on induction of anti-cancer effects by 9 "killer cells" (T cells; natural killer cells and 10 macrophages) with or without using antibodies against malignant cells, as well as agents to neutralize negative regulators of the immune system. Other agents, small molecules or 11 oncolytic viruses that can activate or block cellular signals that can block proliferation of malignant cells or agents designed to reduce the blood supply to malignant tissues can also prevent or minimize progression of the malignant process. Immediately after a person has completed conventional treatment for cancer, blood cancer or solid tumor, when patient’s general condition and immune status are still preserved seems to be the optimal timing for adding additional treatment against chemotherapy-resistant cancer cells, including cancer stem cells that are a priory resistant to available anti-cancer treatment.
Based on the above, Dr. Slavin prefers to inject cancer killer cells into the cancer patient at an early stage of the disease, when cancer cell number is minimal, because killer cells, especially if used together with anti-cancer antibodies, seek out and find any cancer cells which may have escaped surgery and survived the first line chemotherapy. Killer cells obtained from any intentionally mismatched donor are most effective in killing cancer cells but their anti-cancer effects are short acting because these cells that are not an exact match with the patient are consistently rejected. This is why such treatment is most effective and suitable only for patients with minimal residual disease. If tumor load is high, prior tumor 12 debulking is essential and this is why clinical application of such treatment should be best applied at an early stage of the disease, as soon as a stage of minimal residual disease is accomplished, when response to conventional anti-cancer therapy is optimal.
Unfortunately many people with cancer, and their oncologists, may be under the impression that when cancer is no longer visible the patient may be cured, forgetting that cancer lesions of less than 1 millimeter are never visible. Later on, when cancer recurrence occurs, when cancer cells are much more resistant to available anti-cancer treatment, it may be too late to achieve cure. Unfortunately, patients are knocking at death's door before they or their oncologist ever consider treatment other than the traditional radiation and chemotherapy. By that time, when cancer cells become multi-drug resistant, no medical treatment can help them and they are in need of a divine miracle. There are many other ways successfully used at CTCI to control or minimize progression of certain types of cancer using new devices in addition to anti-cancer immunotherapy and use of stem cells and you can find more by visiting their web site at www.CTCIcenter.com or better get even more updated information by approaching CTCI directly by emails or phone call.
When you look at who will benefit from cellular therapy, it is certainly not the pharmaceutical companies. They make billions of dollars each year on each of their chemo drugs. Plus the drugs that a patient needs to counter-act the chemo drugs bring in even more money. Follow the money, find the answers. While Dr. Slavin does not condemn traditional chemotherapy or radiation therapy for treatment of cancer, he says “at the stage of minimal residual disease which can frequently be accomplished by conventional anti-cancer modalities, it may be useful to consider using additional biologic agents focusing on immunotherapy without or when needed with stem cell therapy in order to eliminate or control chemotherapy-resistant malignant cells.
The second type of 13 multi-potent stem cells pioneered and used at CTCI, in full collaboration with Professor chaya Brodie, Bar-llan University, Ramat-Gan, Israel. are the multi-potent 3 mesenchymal stromal cells (MSC). MSCs are completely different from stem cells used for treatment of malignant and non-malignant diseases discussed above. This type of stem cell treatment is not yet approved in the US and Europe, but at CTCI, MSCs are already used for treatment of 14 neuro-inflammatory and 15 neurodegenerative disorders, as well as for treatment of 16 autoimmune diseases on a fully compassionate basis. More recently, MSCs are also being applied for many other indications including local treatment for repair of cartilage in the knee joint, and 17 systemic intravenous applications for treatment of chronic 18 ischemic heart disease, renal failure, and occasionally other indications as well. It is unfortunate that the only stem cell treatments that garner attention in the US, are from fraudulent people who call themselves doctors and who are actually trying to rip off the patients or families who are so desperate to find a cure. In reality, there are many treatments with multiple cures which would completely eliminate the need for on-going drugs and which bring a person back to optimal health with just one or two riskless injections of MSCs.
When asked why the FDA will not approve 19 cell-mediated immunotherapy for treatment of cancer on the one hand, and multi-potent MSCs for regenerative medicine on the other, Slavin responded by saying “Sometimes, in view of what modern biology teaches us, there is no easy way to meet the criteria that the FDA demands; that is, the same treatment for similar patients over and over again under the same circumstances. No two cancer cells are the same and no two patients are the same so there is no reason why treatment should be equal to all patients in need with the same disease.” Slavin went on to say, “Treatment of cancer and many other diseases must be individualized and therefore, in many cases, such treatment strategy cannot meet the demands of regulatory committees and FDA. As far as the use of MSCs for regenerative medicine, the situation is even more complicated because it is a new field not yet considered a standard of care.”
Hopefully pharmaceutical companies will get on the bandwagon and work to develop these practices within their industry. Perhaps then, they could get back to their original focus: Trying to support clinical trials designed to treat, and possibly cure, patients on a fully personalized basis. As it is now, they just keep them sick with one medicine after another for as long as a body can survive with no intention for cure.
Biologic therapy using the immune system and multi-potent stem cells seem to represent Mother Nature’s tool for more effective treatment of malignant and otherwise seemingly incurable non-malignant diseases. Using smarter, rather than more aggressive, modalities for treatment of cancer may eventually be recognized as the Holly Grail of future cancer experts. In parallel, proper use of multi-potent MSCs may prove as the only 20 physiologic modality needed for regenerative and anti-aging medicine.
Glossary
1 Senescence -- the aging process
2 Hematopoietic stem cells - Stem cells which can generate all the circulating blood cells – red, white, platelets – and the entire immune system
3 Mesenchymal stromal cells (MSC) -- Stem cells which can regenerate cells belonging to different tissues by direct differentiation and/or by secretion of factors that can help minimize the damage caused by disease or cancer
4 Adipose tissue – fatty tissue found under the skin and surrounding some major organs; this tissue provides stored energy, insulation and protection
5 Cryopreserved – Storing tissue or cells at extremely low temperature (liquid nitrogen) for use in the future
6 Autologous – From a patient’s own body
7 Immunotherapy -- `the use of the immune system to fight off diseases
8 Monoclonal – cells or products of cells that are formed or derived from a single clone
9 “Killer cells" -- `cells, including T cells; natural killer cells and macrophages that can be used for killing cancer cells
10 Macrophages – a large cell that is present in blood, lymph and connective tissues, removing waste products, harmful microorganisms and foreign materials from the bloodstream. Such cells can also induce direct anti-cancer effects and participate in generating an immune response against cancer.
11 Oncolytic viruses - viruses that can kill cancer cells
12 Debulking -- The process of reducing the volume or number of cancer cells
13 Multi-potent cells – cells that can be used for different functions
14 Neuroinflammatory – inflammation process attacking the nervous system
15 Neurodegenerative – a process that causes degeneration of the nervous system
16 Systemic – a process or treatment that usually is administered intravenously relating to or affecting the whole system
17 Autoimmune – an immune process that attacks patient’s own tissues
18 Ischemic -- A decrease in the blood supply to a bodily organ, tissue, or part caused by constriction or obstruction of the blood vessels
19 Cell-mediated immunotherapy – therapeutic process mediated by the immune system. Such treatment may be induced by lymphocytes or antibodies that direct lymphocytes to their desirable destination
20 Physiologic modality – treatment based on normal biologic mechanisms