New Psychiatric Medications in the Pipeline

The Research and Development Pipeline
Almost 60 million American adults and 300 million people worldwide suffer from some form of mental illness. In the past fifty years we have learned a great deal about mental illness and have seen phenomenal breakthroughs in treatments for mental illness. People who would have been disabled and institutionalized fifty years ago are now able to function in their communities and maintain their independence.
Researching and developing new medicines to treat mental illness is a slow and expensive process. There are about 3,000 medicines of all kinds in some stage of development, from early research to later trials to awaiting approval by the FDA. About 300 medicines in the research and development pipeline are aimed at treating mental illnesses.
Medicines in Development for Mental Illness
Disorder
| # Drugs in Pipeline
| # US population impacted
|
|---|---|---|
Depression and mood disorders
| 71
| 21 million
|
Addictive disorders
| 33
| 25 million**
|
Anxiety disorders
| 38
| 40 million
|
Dementias (including Alzheimer's)
| 90
| 5 million
|
Schizophrenia
| 54
| 2.4 million
|
Attention Deficit/Hyperactivity disorder
| 20
| 1 in every 20 children
|
Developmental disorders (includes autism)
| 6
| 1 in every 110 8 year olds
|
Eating disorders
| 33
| ***
|
Personality disorders
| 2
| not available
|
Premenstrual disorders
| 2
| up to 75% PMS, 5% PMDD
|
Sleep disorders
| 21
| not available
|
Other
| 3
| ****
|
*Some medicines are in development for more than one disorder.
**Alcoholism afflicts 13 million adults and children. 12.5 million Americans are addicted to other drugs. Another 40 million family members, children and victims of drunk drivers are affected indirectly.
*** 0.6% of adult population in the US suffers from anorexia, 1.0% from bulimia, and 2.8 percent from a binge eating disorder. 32.7% overweight, 34.3% obese, 5.9% extremely obese.
****Schizoaffective disorder 0.2% to 0.5% - a combination of symptoms of schizophrenia and mood disorder
Examples of Medications Now Being Tested to Treat Mental Illness
Schizophrenia
- A medicine to treat both positive and negative symptoms of schizophrenia without the negative side effects that are common with current antipsychotic medications.
Schizophrenia is the most common type of psychotic disorder, and is very disabling. A person with a psychotic disorder loses touch with reality. In addition to psychotic symptoms of hallucinations and delusions, a person with schizophrenia experiences disturbances in thinking, emotional reactions and behaviors.
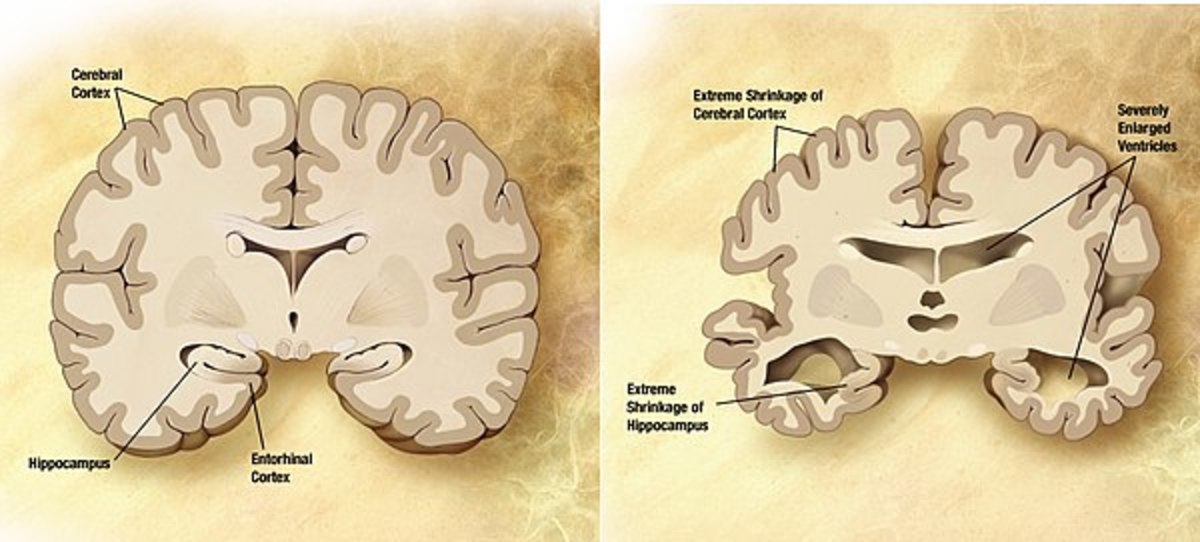
Alzheimer's Disease
- A medicine to treat Alzheimer’s disease that removes beta amyloid protein from the brain that will prevent or reverse progression of Alzheimer’s.
Early signs of Alzheimer’s include a decreased attention span, poor concentration, personality change and forgetfulness. As the disease progresses there is a loss of computational ability, problems with finding words, and difficulty with ordinary activities.In later stages, there is severe memory loss, complete disorientation, social withdrawal, loss of independence, and finally death. Alzheimer’s is the seventh leading cause of death in the US.
Three years after Alzheimer’s disease begins, half of untreated patients are placed in nursing homes, compared with only 11% of patients who receive treatment. One study found that if a new treatment is found that can delay the onset of Alzheimer’s, Medicare and Medicaid spending on patients with Alzheimer’s would be reduced by more than $100 billion a year by 2030. If no new medicines are found, Alzheimer’s disease could affect 13.5 million Americans by 2050.
The Drug Development and Approval Process
The US system for new drug approvals is the most rigorous in the world. On average, it takes 10-15 years and $1.3 billion to get a drug from the laboratory to a patient. The process involves the following steps.
Preclinical Testing
The drug company demonstrates through lab and animal studies that the compound targets disease and is safe.
Investigational New Drug Application (IND)
After preclinical testing, the company files an IND with the FDA (Food and Drug Administration) to begin to test the drug on people. The IND shows results of previous experiments and provides information about the compound and how it works in the body, the proposed studies, and findings from the animal studies. Annual progress reports are submitted to FDA.
Clinical Trials – Phase I
Tests involve 20-100 normal, healthy individuals. The studies determine how a drug acts in the body and how long the effects of the drug last.
Clinical Trials, Phase II
This phase involves drug trials with 100 to 500 patients with the disease the drug is intended to target. The effectiveness of the drug is studied as well as any early side effects.
Clinical Trials, Phase III
Phase III involves 1,000 to 5,000 patients. Doctors monitor patients in clinics and hospitals to see if the drug is working and to identify any adverse effects.
New Drug Application (NDA)/Biologic License Application (BLA)
When the clinical trials are completed, the company analyzes the data from the trials. If the data supports that the drug is effective and safe, the company files an NDA or BLA with the FDA. The application includes all of the data and scientific information the company has gathered. The applications are typically 100,000 pages or more and take about 13 months to review. There were 25 new drugs approved in 2009. The average review time was 13.3 months.
Approval
Once approved, the new medicine becomes available, and physicians begin to prescribe them to patients. The company continues to send reports to the FDA, including reports on any adverse reactions to the drug. For some medicines, FDA requires additional trials (Phase IV) to evaluate long-term effects of the medicine.
Other Drugs in the Development Pipeline for Mental Illness
Anxiety (Social Phobia) – One medicine in the pipeline for anxiety is part of new class of psychotropic pherines. The drug is administered in an intranasal spray. It acts very rapidly on nasal chemosensory neurons that act on the hypothalamic-limbic system in the brain. The clinical trials demonstrated that it improved social performance and social interaction anxiety within 10 minutes of administration.
ADHD – A new medicine in the developmental pipeline targets specific receptors (NNRs), which release several neurotransmitters and control the flow of neuron signaling. In clinical trials it demonstrated improvement in ADHD symptoms, improved academic and occupational functioning, and improved quality of life.
Cocaine Addiction – Currently there is no medication treatment for cocaine addiction. A vaccine is in development that might be an effective treatment. The vaccine induces cocaine-specific antibodies that bind to cocaine in the blood and block its uptake in the brain. The pleasure response is altered and reinforcement of addictive behavior is reduced, allowing patients to interrupt the addiction cycle.
Depression with Anxiety – This medication is a result of recent discoveries connecting neurogenesis to central nervous system diseases. Neurogenesis is a process in which stem cells in the brain produce new brain tissue, including neurons. A neurogenic treatment can help neurons differentiate and survive. Since it does not affect serotonin levels, it could eliminate some of the negative side effects of typical antidepressants that act on serotonin.
Insomnia – A new medication in the development pipeline targets the protein orexin in the brain. Orexin regulates the sleep-wake cycle. By interacting with hormones, the drug could reduce or eliminate some of the side effects of current insomnia treatments. This medication induces sleep without affecting REM sleep.
Obesity – A medicine is in the pipeline that stops the pleasurable response to sugary and fatty foods and may contribute to weight loss in obese patients.