The Federal Food, Drug and Cosmetic Act – What’s with the FDA?
In 1938, Congress enacted The Federal, Food, Drug and Cosmetic Act. This legislation granted the FDA with authority to oversee the safety and regulation of drugs. Although the legislation has been amended several times, a personal use policy still exists within the legislation. This policy outlines a suggested lax enforcement for individuals importing prescription drugs from foreign countries for personal use by granting waivers on a case-by-case basis. In recent years, however, these policies have been tossed aside.
Author's Note:
This hub is not designed to give legal advice. It is designed to point out that the FDA has had policies in place allowing individuals to import prescription medications from foreign countries for personal use; however, these policies seem to have changed even though the legislaion has not been altered. This is probably the reason why there are inconsistencies and/or unclear statements within various governmental agencies on the legalities of obtaining prescription medications from foreign countries.
Denying Access to Affordable Prescription Drugs Became John Horton's business
- The Conspiracy to Deny Americans Affordable Prescription Drugs
Find out how John Horton, founder of Legitscript, used his position and his policy changes in the Bush Administration to launch LegitScript, a multi-million dollar business.
Inconsistencies that cause confusion regarding the personal use policy:
There seems to be differences in opinion by a wealth of different people and organizations on whether or not it is legal to import prescription medications from foreign countries. This is especially true with medications imported from Canada since every aspect of prescription drugs and drug safety are similar to U.S. standards.
Currently, the FDA maintains that importing prescription drugs from foreign countries, including Canada, is illegal, generally.
There are, however, legitimate pharmacies in Canada that indicate Americans are allowed to order a 90-day supply of medication under U.S. law. This is true with CIPA (Canadian International Pharmacy Association), a Canadian association of licensed retail pharmacists. Since 2010, CIPA has been the official verifying organization for all pharmacies that want to advertise on Google Canada, but it is not contracted to provide this service in the United States.
Google relies on CIPA to verify online pharmacy advertisements in Canada
However, the National Association of Pharmacy Regulatory Authorities (NAPRA) in Canada maintains that it is against U.S. law for Americans to fill prescriptions through Canadian pharmacies; although NAPRA, like many governmental agencies in the U.S., does use words such as "generally" and "typically" when (not) elaborating on these legalities.
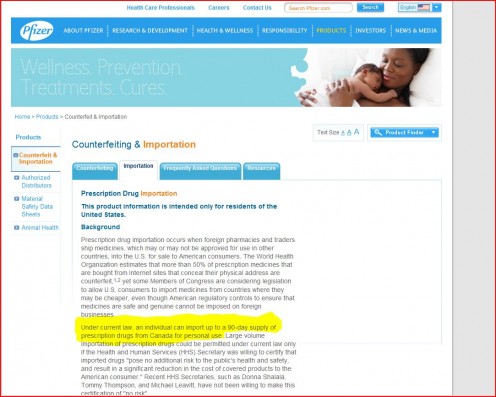
To make matters even more confusing, drug-giant Pfizer states on its website that Americans are allowed to import medications from Canada.
AND WHAT'S WITH PFIZER?
- U.S. Customs and Border Protection
In virtually all instances, individual citizens are prohibited from importing prescription drugs into the United States.
Government agencies like the DEA (Drug Enforcement Agency) and CBP (U.S. Customs and Border Protection) seem to skirt around the legality issues with very general and broad statements about importing prescription drugs.
Perhaps the website of U.S. Customs and Border Protection (CBP), a division of the Department of Homeland Security, is the best example of this generalization:
The Federal Food, Drug, and Cosmetic Act (the Act) prohibits persons from importing into the United States any prescription drug that has not been approved for sale by the United States Food and Drug Administration (FDA), or which is adulterated or misbranded within the meaning of the Act. Moreover, in those instances where a United States manufacturer makes an FDA-approved prescription drug and sends it abroad, the Act also prohibits any person other than the original manufacturer from importing the drug back into the United States. Thus, in virtually all instances, individual citizens are prohibited from importing prescription drugs into the United States.
Okay, so what’s the catch? Under what circumstances are American consumers allowed to order prescription drugs from foreign countries?
Obviously, if the drug manufacturers were kind enough to ship the medication back to American consumers after selling it to another country (at a cheaper price), it would not only be legal to receive imported prescription drugs from foreign countries, but consumers would still be paying less despite medications being exported and imported then exported and imported, again. However, I highly doubt that any of the drug manufacturers will be offering to do this for Americans who desperately need affordable prescription drugs.
Another circumstance is under the 1988 Orphan Drug Act. This amendment to the Federal Food, Drug and Cosmetic Act allows individuals to import unapproved drugs for certain medical conditions. This amendment was a result of the lack of approved, available medications for HIV patients in the United States. Other countries were developing and approving drugs for the treatment of HIV, and the United States was far behind.
The FDA still maintains unapproved drugs are allowed to be imported under these circumstances:
--if the intended use is for a serious condition for which effective treatment may not be available domestically
--if the product is not considered to represent an unreasonable risk
--if the individual seeking to import the drug affirms in writing that it is for the patient's own use and provides the name and address of the U.S.-licensed doctor responsible for his or her treatment with the drug or provides evidence that the drug is for continuation of a treatment begun in a foreign country
--if the product is for personal use and is a three-month supply or less and not for resale, since larger amounts would lend themselves to commercialization
--if there is no known commercialization or promotion to U.S. residents by those involved in distribution of the product
http://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm143561.htm
The FDA further states:
That means if you buy your high blood pressure or other medication from a foreign country because it's cheaper--even though a drug with the same name is approved for sale in the United States--generally the drug will be considered unapproved and the FDA's personal use guidance will not apply. The Drug Enforcement Administration has additional requirements for controlled drugs.
When the personal use policies started to change:
In 2003, governmental agencies stepped up enforcement, including seizing imported prescription drugs from Canada. These seizures had members of Congress asking the FDA why the policy of allowing imports of prescription medications for personal use had changed. FDA officials claimed policy had not changed. Packages of prescription medications were released to consumers a month after Congress submitted a letter to the FDA requesting an explanation.
LA Times journalist, Lisa Girion, reports: The crackdown prompted seniors and members of Congress to demand explanations for why the government was not following a Food and Drug Administration policy that allows people to mail-order or carry across the border a 90-day supply of prescription drugs from licensed Canadian pharmacies.
Prescription drugs seized and released after Congress complained
- More Medicines From Abroad Seized - Los Angeles Times
The U.S. government apparently is stepping up seizures of cheap drugs ordered by Americans -- mainly seniors -- from abroad, Canadian pharmacies say.The pharmacies, which sell drugs by mail and over - Seized Drugs Being Released - Los Angeles Times
Amid mounting criticism of their crackdown on mail-order medications, customs officials have begun releasing hundreds of seized packages to consumers, Canadian pharmacies and U.S. lawmakers said
Section 804 of The Act: Personal Use Policy
There is a large section in the Act—Section 804—that addresses imports of prescription medication by individuals for personal use. This is why governmental agencies often skirt around the legalities of drug importation. Even the above statement by the FDA includes the word “generally”.
In Section 804, Congress suggests: enforcement against individuals be taken only when the importation poses a significant threat to public health; authorities should exercise discretion when the importation is clearly for personal use; there doesn’t appear to present an unreasonable risk to the individual.
The Act, in section 804, further addresses imports, specifically from Canada: waivers may be granted to individuals when: the drug imported is from a licensed pharmacy for personal use by an individual, not for resale, in quantities that do not exceed a 90-day supply; is accompanied by a valid prescription; is a seller registered with the Secretary; is a prescription drug that has been approved; and a prescription manufactured in a location that is registered.
Lastly, at the very beginning of Section 804 of the Act, it reads:
(b) REGULATIONS.--The Secretary, after consultation with the United States Trade Representative and the Commissioner of Customs, shall promulgate regulations permitting pharmacists and wholesalers to import prescription drugs from Canada into the United States
It has always been widely accepted that Canada’s practices in regards to prescription drugs—regulation, handling, labeling, etc—are very similar to U.S. practices.
And the above suggests that there would have been, by now, some measures put into place that would have allowed Americans to safely purchase prescription medications from Canada.
VIPPS, a voluntary accreditation process
- General Information about VIPPS - NAPRA
The VIPPS program was developed in 1999 by the National Association of Boards of Pharmacy (NABP) in the United States, in response to public concern about the safety of pharmacy practice offered through the Internet. In 2003, NAPRA entered into a lic
Shhh...Don't tell anyone, but there are safety measures...
Well, this was done back in 2003 when the NABP (National Association of Boards of Pharmacy) entered into a licensing agreement with Canada’s NAPRA. The U.S. has, since then and currently, been accrediting online Canadian pharmacies the same way it accredits pharmacies in the U.S.
However, in order to qualify for the accreditation, online pharmacies located in Canada are unable to ship prescriptions to American consumers. Additionally, Canada’s NAPRA recommends to Canadian citizens that only VIPPS-accredited online pharmacies be used to obtain prescription medications online.
This, most likely, is due to the fact that the FDA, beginning around 2003, began to send warning letters to Canadian pharmacies threatening to take legal action against the pharmacies and employees of the pharmacies. One such warning letter from the FDA reads, in closing:
If you do not promptly correct your violations, FDA may take legal action without further notice. Possible actions include seizure and/or injunction. Further, federal agencies are advised of the issuance of all Warning Letters about drugs so that they may take this information into account when considering the award of contracts.
Shortly after the FDA warning letters were sent out, drug companies stopped filling orders to those Canadian pharmacies. This caused the beginning of the drug shortage problem in Canada.
It seems that the FDA's policy has, indeed, changed. The FDA has become so strict on the personal use policy/guidance that the agency, perhaps, has been the sole cause of the black market, rogue pharmacies, and counterfeit drugs sold to Americans.
So, who's behind it and how did it happen?
The Conspiracy to Deny Americans Affordable Prescription Drugs.