Future Car - Electricity & Battery Technology
Electrical Storage - Batteries
Though the only mention of "future" or "car" in this hub is in this and the next paragraph, it is with an eye toward the future car that the hub was written.
With so many new cars being designed to run solely on electricity or generate electricity within the car itself, I thought it important to discuss;
- what electricity is
- the two forms of electric flow or current (direct and alternating)
- how batteries work to store electricity
- the advantages and disadvantages of battery storage methods
What electricity is takes the most explaining and involves two theories; electrical and atomic. The two forms of current are much easier to explain because they involves "flow." Battery storage methods require all three of these explanations, but once made the methods each battery type employees is that much easier to explain. Finally, the materials used to make these batteries and the reason these materials are chosen is that much more easily explained by delving into electrical and atomic theory and electrical flow.
Please note that the term "hybrid" is thrown around a lot these days. Often the car called "hybrid" really isn't. Check out my hub "Future Cars - Hybrid Types" for an explanation.
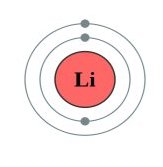
Lithium Atom
Click thumbnail to view full-size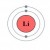




Electricity
To explain what electricity is requires an understanding of the electron theory. The electron theory, in turn, requires an explanation of atomic theory so we'll start there.
Atomic Theory
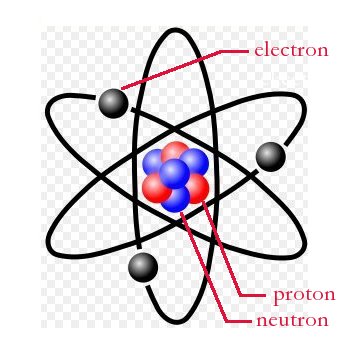
Every atom consists of three main particles, broken into two groups. The center (first group) of an atom consists of protons and neutrons. A proton has an electrical charge that is positive. The neutron, as the name implies, has no charge; it is neutral.
Orbiting the nucleus (second group) are one or more electrons. An electron has a charge that is negative. Typically, an atom has an equal number of these charged particles. One proton for every electron.
An atom with this arrangement is said to be electrically neutral. As such, an atom with a "balanced" number of protons and electrons has no net charge; it is electrically neutral. If an atom gains or loses an electron it will have a net positive or net negative charge.
Such an atom is called an ion. Ions have more or fewer electrons than protons in the nucleus making them ideal candidates for electrical transfer.
Electron Theory
Since electrons orbit the nucleus and the nucleus contains the protons, it is much "easier" to strip off or add to the number of electrons orbiting the nucleus. Once an addition or subtraction happens an imbalance is created and the atom acquires an electrical charge; either positive or negative.
If an atom has more electrons than neutrons then the atom has a negative electrical charge. If an atom has less electrons than neutrons then the atom has a positive electrical charge.
Elements that Conduct Electricity
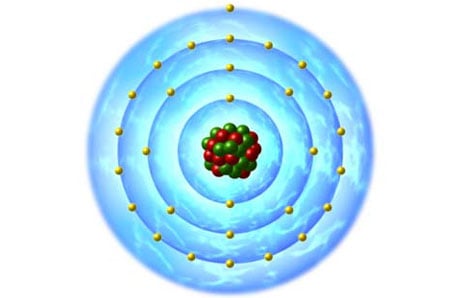
There are a number of elements that are ideal for conducting electricity. Three in particular are used often in electrical/electronic devices. One less often than the other two. These elements are;
- copper
- silver
- gold
All three of these elements have a single electron in the outermost orbit of their atoms.(see images above right) It is believed that these single atoms have electrons that are easily "nudged" out of their orbits by incoming electrons. This way there is a constant flow, under current, of electrons from one atom's orbit to the next; thus they conduct electricity fairly easily.
Copper is the most commonly used of these elements with gold used less often and silver infrequently. The reasons for the first two are pretty obvious. Copper is cheaper than gold, but it corrodes in air. Gold doesn't corrode, but is expensive. For this reason electrical contacts that require gold are typically plated; not solid. Silver is both expensive (when compared to copper) and it corrodes.
Current
Current is the flow of electricity, either in one direction or oscillating between two directions. Electricity that flows in one direction is called Direct Current. When it flows in one direction and then another (there are only two) it is called Alternating Current.
Electric currents that occur in a battery involve the flow of positive and negative ions. Electric current is power that can be converted to heat, light, or used to impart a rotational force. Current is said to move electrons from a region with a negative charge to a region with a positive charge; thus electrical flow runs from negative to positive. Examples of direct current use are; flashlights, remote controls, cell phones, and cordless tools.
Electrical current that travels in one direction and then reverses direction is called alternating current. How often that directional change occurs is called "frequency" often measured "Hertz*." So, for example, a current that changes direction sixty times a second has a cycle of sixty (60Hz) Hertz. Examples of alternating current use are things that are plugged into the wall. Such devices would include a vacuum cleaner, refrigerator, microwave, or toaster.
Direct current can be transformed into alternating current and alternating current can be transformed into direct current.
* Named for Heinrich Hertz.
How Electrochemical and Rechargable Batteries Store Electricity
Batteries
First, batteries are all direct current. There is no such thing as an alternating current battery.
There are two basic types of battery. Electrochemical and rechargeable. Electrochemical batteries produce electricity via a chemical reaction. Once the chemicals that cause this reaction are spent the battery no longer works. A rechargeable battery, on the other hand, can take a charge of electricity from an external source. This involves moving electrons from one part of the battery to another. When a rechargeable battery is discharged electron flow goes in one direction; when the battery is recharged that flow goes in another direction.
Electrochemical Battery
An electrochemical battery only produces electricity when there is a demand placed upon it. This "demand" can even be conduction through air (depending on the humidity), though that conduction is very low. For that reason electrochemical batteries can go unused for years and still retain a charge. As electrical demand is placed on one of these batteries the chemicals that cause the production of electricity are used up. This means that an electrochemical battery has a finite life (when in use) and must be replaced from time to time.



Rechargeable Battery
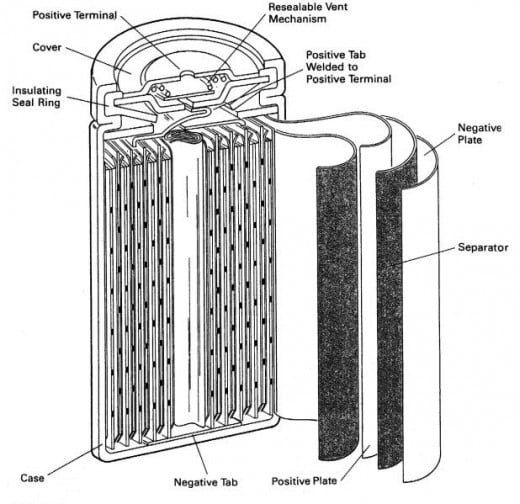
Even though a rechargeable battery also uses chemicals it does so in a different way. The chemistry is used primarily to move electrons from one place in the battery to another. The chemicals do eventually wear out, but at a far slower rate than a standard electrochemical battery. When a rechargeable battery is put to use electron flow moves from one part of the battery to another. When the battery is recharged that electron flow is reversed. Common rechargeable batteries include;
- Lead-Acid batteries (common car battery)
- Nickle Cadmium (NiCad)
- Nickle Metal Hydride (NiMH)
- Lithium Ion (Li-ion)
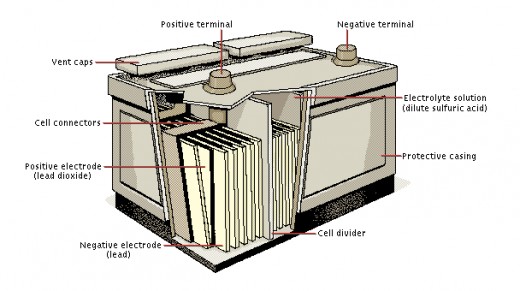
Lead-Acid Battery: The first rechargeable battery was invented by Frenchman Gaston Plante in 1859. This is the lead-acid battery so commonly found in gasoline or diesel engined cars. The battery consists of alternating plates of lead and lead dioxide. Between these plates is a solution of sulfuric acid. As the battery is used (discharged) the lead on the lead-plate combines with the sulfur to create lead sulfate. This process also frees up an electron. Then the lead dioxide, some hydrogen ions, sulfate ions, plus electrons from the lead plate, work together to create lead-sulfate and water on the lead dioxide plate.This reaction is completely reversible by applying more voltage to the battery than it produces. The excess voltage (about 13.4 volts) breaks up the lead-sulfate, combines the sulfate with water (producing sulfuric acid) and rebinds the previously free electrons back to the lead plate.
These lead-acid batteries last a considerable amount of time, have no "memory", and though heavy are quite reliable. They are also quite cheap (despite what you pay for them) to produce.
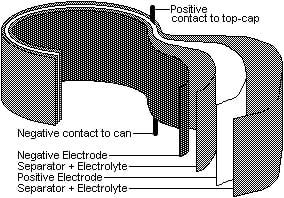
Nickel Cadmium Battery: These batteries are similar to the lead-acid type except that electrodes are nickel-hydroxide (instead of lead-dioxide) and cadmium (instead of lead), with potassium-hydroxide (instead of sulfuric-acid) as the electrolyte. NiCad batteries suffer from a "memory effect" in that if the battery is discharged to a certain level then fully recharged after x number of recharges the battery will stop discharging once it reaches that point again. e.g. if the battery is routinely discharged to 50% of capacity it will stop producing current when it reaches that capacity again after a recharge. On the upside NiCad batteries lose little energy to the air, do well in the cold, and can take a trickle charge.
Nickel Metal Hydride:This battery is similar to the Nickel Cadmium except that it uses a hydrogen absorbing alloy of metals (this alloy is a metal hydride; good at storing hydrogen) instead of cadmium at the negative electrode. NiMH batteries have two to three times the storage capacity of NiCad batteries. They have a long shelf-life, wide operating temperature range, and like NiCad do not "leak" a great deal of energy to the air. A.K.A. self-discharge.
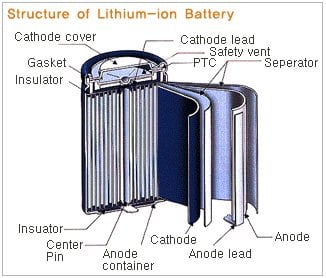
Lithium-Ion: The lithium ion battery has the three same basic internal structures; a cathode, anode, and electrolyte. In the case of the Li-ion battery the cathode is either lithium cobalt oxide, lithium iron phosphate, or lithium manganese oxide. The anode is typically graphite or a lithium graphite alloy. When Li-ion cells are discharging, the lithium is extracted from the anode and inserted onto the cathode. When the Li-ion cell is charging the lithium-ions reverse flow; depositing ions onto anode from the cathode. The electrolyte is typically a lithium salt.
Lithium-ion batteries, unlike NiCads, do not suffer from a "memory" effect, do not self-discharge readily (only about 5% per month), and can be charged and discharged hundreds of times.
Energy Density: Energy density is the amount of energy storage per kiogram of weight. By this measure the following battery types store; (source for table below allaboutbatteries.com)
Energy Density by Battery Type
Battery Type
| Energy Density
|
|---|---|
Lead-Acid
| 30~41 Watthour (Wh) per Kilogram (Kg)
|
Alkaline
| 110 Wh per Kg (not rechargeable)
|
Nickel Cadmium
| 39 ~ 42 Wh per Kg
|
Nickel Metal-Hydride
| 95~ 100 Wh per Kg
|
Lithium-Ion
| 130~150 Wh per Kg
|
An electrochemical battery can be created from a lemon which has inserted into it a zinc galvanized nail and a copper coin. Because of the acid in the lemon electrons will gather at the zinc nail and, when connected via wire, flow to the copper coin. A lemon battery produces slightly less than one volt.
Disadvantages
Each battery type has distinct advantages and disadvantages. The advantages are discussed with each type above. Each battery type listed below is in the order of their introduction to the public and also in the order of cost (and therefore price paid for) to produce.
The disadvantages are;
- Lead-acid batteries
are heavy, involve some very caustic chemicals, have a low energy
density, and have a limited life. Though the lead is highly recyclable
the sulfuric-acid is problematic and the release of hydrogen gas during
recharge is quite dangerous.
The low energy density makes them a very poor choice in electric or hybrid cars. The weight of the battery combined with it's low energy density make the use of lead-acid batteries in electric/hybrid cars nearly unworkable. - Nickel Cadmium Battery
has a low energy density, suffer from a "memory" effect, and can be
damaged beyond use if fully discharged. They also contain toxic
chemicals which makes "household" disposal a problem.
The same problems with the lead-acid battery as a primary energy repository for electric/hybrid cars is nearly the same as for NiCad batteries. - Nickel Metal Hydride Battery
has a (now) low energy density compared to Lithium-ion. Chargers must
apply "over voltage" which in turn makes them dangerous without a
charger designed just for this battery type. Such a charger monitors
the state of charge constantly. They can be trickle charged however
this has to be a "pulse" charge which also requires a specially
designed charger. If improperly charged these batteries can explode or
catch fire.
These are the batteries that were used in the in the first Toyota Prius and Honda Insight. The 2010 models of these cars, along with scores of others, will rely on the next battery type below. - Lithium-Ion Battery begins to degrade
immediately after manufacture which means they have a very definite
useful life of two or three years. They are
temperature extreme intolerant and high temperatures will damage them
permanently. They cannot be completely discharged; this will ruin them.
Charging and discharging must be coordinated with an on board
computer...thus making them that much more expensive. Finally, a
lithium-ion battery with a short-circuit between anode and cathode could overhead and burst
into flame.
These are the batteries of choice for the next generation of hybrid electric cars and electric only cars. The limited life-span will make this an "interesting" choice.
This is the fourteenth in a series of future cars.
Future Car Series
- Future Car - Hybrid Types
With all the talk of - Future Car - Ford's Hydrogen I.C.E.
I.C.E. stands for Internal Combustion Engine. Ford is currently looking at creating a car that will bridge the gap between gasoline power and the very likely future fuel; hydrogen. The I.C.E. burns hydrogen... - Future Car - Chrysler EV Series
EV stands for Electric Vehicle. Much to everyone's surprise Chrysler recently unveiled an all electric vehicle called the Dodge Circuit EV. The Circuit will be an all electric sports car vaguely reminiscent... - Future Car - Chevrolet Volt
The Chevrolet Volt is a range extended electric that can be plugged into a standard wall outlet (120V). The vehicle to be produced by the General Motors Chevrolet division. It is expected to be launched as a... - Future Car - The Problems with Hydrogen
Hydrogen is the lightest element in the periodic table and the first listed. It is a colorless, tasteless, odorless, non-metalic gas that is highly flammable. Hydrogen can be ignited in an air (or oxygen)... - Future Car - Subaru
Subaru is the western spelling for the Japanese word for - Future Car - Peugeot / Citron
Peugeot and Citron is now known as Peugeot Citron. The companies are now combined though each is marketing and selling it's own particular models. If Citron continues as a viable company remains to be... - Future Car - Nissan Leaf
Founded in 1931 as Datsun, Nissan Motor Company is a publicly traded company and one of the largest in Japan. In 1999, Nissan allied with Renault S.A. of France, when that company bought a forty-four point... - Future Car - Tesla
Tesla1 Motors was founded in 2003 by Martin Eberhard, Marc Tarpenning and Ian Wright in an attempt to build upon and market an all electric car based on the TZero AC propulsion car. The propulsion system, in... - Future Car - Aptera
According to Paul Wilbur, Chief Executive Officer of Aptera Motors, Aptera is Greek for - Future Car - Opel
Opel, in coopreation with General Motors, has introduced the Ampera (or is it Flextreme?) as a diesel plug-in series hybrid concept car. It can travel thirty-four (34 mi) miles on its lithium-ion battery... - Future Car - Renault
Renault, a French automaker, was founded in 1898 by Louis Renault, and his brothers Marcel & Fernand along with friends Thomas Evert and Julian Wyer. While Louis handled design and engineering Marcel and... - Future Car - Electricity & Battery Technology
Though the only mention of - Future Car - Electric Motors
Introduction Though there are only brief mentions of - The Future of the Car
The car of tomorrow promises to be radically different than what we are driving today. Pardon the Science Fiction wording please.There are many reasons for these advances; Computing and programming A...