Methods and Ways to separate mixtures of components in the lab Distillation, Extraction, Gas chromatography, Adsorption
[EDITOR'S NOTE: Other exam topics are: Macromolecules and Polymers, Acid/Base reactions, Redox reactions, environmental chemistry (ozone depletion), Molecules/Salts/Metals/Atomic lattice, Equilibrium equations and organic chemistry]
Extraction
Extraction: Salts with different solubility are solved in water.
Evaporation
Evaporation: Two big glass plates are above a black with seawater filled basin. Since the sunrays heat up the black basin very fast, the water evaporates, rises and condenses on the glass plates, where it will flow down and be collected. This is only worth doing for small amounts of seawater and if a big enough area is available.
Distillation
Distillation: Hot water vapour heads through a tank, which is filled with seawater. The seawater is heated up by the hot water vapour and the water in the seawater evaporates. The water vapour heads to the next water tank and the process is repeated several times. The pressure inside the pipes also increases, so the evaporation process is faster. The water vapour is collected in the end.
Permeability
Permeability: Seawater is pressed through a very narrow membrane. It is used for small amounts of water and the particle size of water must be smaller than the particle size of the salt.
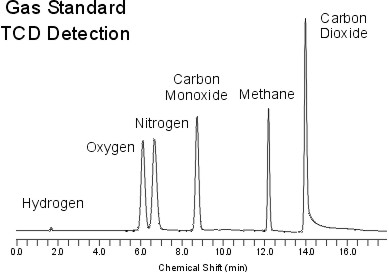
Gas chromatography (GC)
Gas chromatography (GC): A carrier gas (e.g. H2O, He, N etc) (mobile phase) streams continually through a pillar (stationary phase). A liquid probe is injected into the beginning of the pillar. The probe is carried by the carrier gas through separation columns. The components will go through it faster or slower to the end. At the end, there is a detector that shows when which molecule has reached the end. The induced signal is translated on paper, thus producing graphs.
Thin-layer chromatography
Thin-layer chromatography: A small disc represents the stationary phase. The molecules adhere differently well to it. So, there are molecules, which travel a long distance on it and other do not travel so far (because they are adhered to the disc very well).
Adsorption
Adsorption: Activated coal is used. It has an extremely big surface with hollows where molecules can be captured. This yields pure H2O.