Redox Reactions (oxidation, reduction), process of making aluminium and Galvanic, Alkaline and Zin-Carbon cell and more
[EDITOR'S NOTE: Other exam topics are: Macromolecules and Polymers, Acid/Base reactions, Redox reactions, environmental chemistry (ozone depletion), Molecules/Salts/Metals/Atomic lattice, Equilibrium equations and organic chemistry]
Definition of redox reaction
A redox reaction is the transfer of electrons, where one of the reactants involved will lose charge and the other one will gain charge. Note that acid / base reactions transfer protons (H+) and not electrons.
A redox reaction will happen spontaneously if the “S-slide” rule is fulfilled. The higher the atom on the left side is compared to the atom on the right side, the steeper the “slide” will be and the easier they react.
Oxidation numbers
The oxidation number tells the amount of electrons of an atom in a molecule, which it would have if all the other ligands were removed.
The rule is simple. One takes the valence electrons of a molecule (which can be found on the table of elements) and substrates its formally assigned number of electrons. The formally assigned number of electrons depends on the electronegativity of the atom itself and the atom it shares the electrons with. If the atom has a higher electronegativity than the other atom it makes a bound to, then it will “get” its electrons and the formally assigned electron number will increase. The number is represented with Roman numbers.
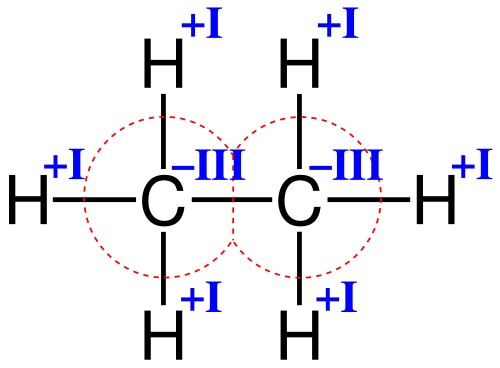
In the example above, one can see that the oxidation number of carbon is -3 (-III). Its valence electron number is 4 (IV). Three more electrons are assigned to it, as it has a higher electronegativity than hydrogen (electronegativity of hydrogen: 2,1; electronegativity of carbon: 2,5). So, the total amount of assigned electrons is four (which it already had before) plus three of hydrogen (which it “took away”). By subtracting, it gives -3 (-III).
A lot easier are ions and molecular compounds like hydrogen (H2), oxygen (O2) etc. The oxidation number of ions is simply its charge. The oxidation number between molecules such as hydrogen, oxygen etc. is always 0.
With oxidation numbers, redox reactions can also be carried out.
Example: C (-I) ->C (+III) + 4e- (oxidation)
Carbon has been oxidized, as it has given off four electrons.
Example 2: Mn (+VII) + 3e- ->Mn (+IV) (reduction)
Manganese
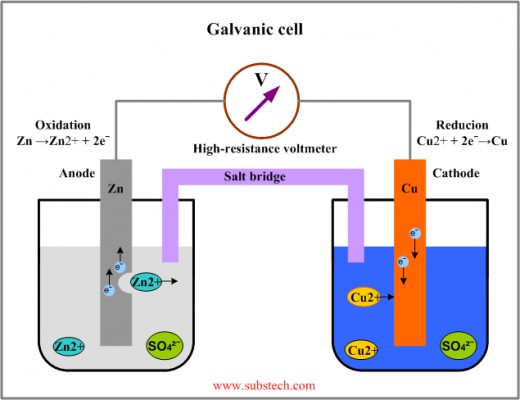
Galvanic cell
The right-hand side has a copper cathode, which is in a solution of nitrate ions (NO3-) and copper ions (Cu2+). The copper ions will be reduced, since the electron flow goes through the copper anode and the solution. The copper ions will form a layer of copper on the anode. The anode does not have to be necessarily made of copper. It can be another non-reactive metal like platinum. The electron flow continues through the salt bridge, which has on each opening a cotton plug in order to prevent any unwished ions going into the salt bridge. The salt bridge is sodium ions (Na2+) and nitrate ions (NO3-). Without these ions, the electrons could not be able to find a way to the left-hand side. The left-hand side has a solution of zinc ions (Zn2+) and nitrate (NO3). The zinc is oxidized there and the zinc anode will be eventually used up as it releases steadily new zinc ions and thus also releasing electrons which go upwards. The electron flow continues through a voltmeter, which measures the voltage of the electron flow respectively the cell potential (energy!). The cycle is closed when the electrons reach the copper cathode and the process starts from the beginning again.
Cell potential
The cell potential is the energy, which a cell has in a spontaneous reaction. It can be easily calculated by subtracting the normal potentials of the reduction reaction and the oxidation by the following formula (the normal potential of a reaction can be normally read off on a redox reaction table):
Normal potential (V) = (REDUCTION) – (OXIDATION)
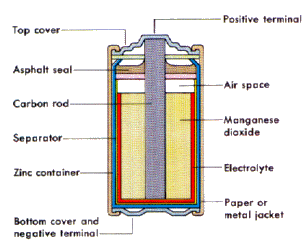
Carbon-Zinc cell
This cell type is separated by a mere separation paper in order to prevent any unwished reactions. The inner rod is a graphite (carbon) rod (negative end) which stays unreactive. In between the graphite rod and the separation paper is manganese dioxide (MnO2), which is capable of conducting electrons from the positive to the negative end. Manganese dioxide (MnO2) will be oxidized during this process. Right after the manganese dioxide, there is an electrolyte which is responsible for conducting the electrons to the manganese dioxide. In this case, the electrolyte is ammonium chloride (NH4Cl [aq]). The outer ends are the positive ends which consist of zinc, reducing it.
Oxidation: Zn <=> Zn2+ + 2e-
Reduction: MnO2 + e- + H+ <=> MnO(OH)
Alkaline battery
The reactions happening are actually the same as in a carbon-zinc cell. The only difference is that the electrolyte is not ammonium chloride (NH4Cl), but potassium hydronium (KOH) (aq). This electrolyte forms a soluble compound Zn(OH)42-, which prevents the death of a battery or cell, since there is no crust formed.
Build-up of an aluminium electrolysis
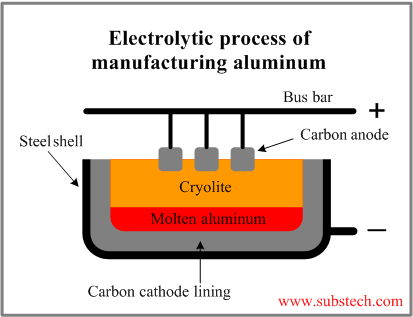
Aluminum electrolysis cell
The aluminium electrolysis cell was the first invention, which allowed a cheap mass production of aluminium.
As it can be seen in the picture, the anode (positive end) is made out of huge carbon beakers, which reduce oxygen ions. The lower part is molten aluminium, where aluminium is oxidized. The aluminium comes from bauxite, which is a mixture of several molecules and since aluminium is very difficult to be gained from bauxite, a catalysator is used. It is cryolite, which is a salt (Na3AlF6).
The advantage of this way of producing aluminium is that the reduction needs oxygen and oxygen is everywhere anyway. The disadvantage of this is that huge amounts of energy are used in order to produce aluminium.
Reduction: 2O2- -> O2 + 4e-
Oxidation: Al3+ + 3e- -> Al
Principle of Rechargeable batteries
Principle: The principle is quite easy. By charging a battery, the electron circuit (flow) is reversed, and thus, the redox reaction is reversed. This is possible, because the compounds, which are formed during normal activities of a battery, stick to the electrodes (anode and cathode). Logically, the reversed electron circuit will go through these electrodes and reduce respectively oxidize the compounds.
Picture of a PEM fuel cell
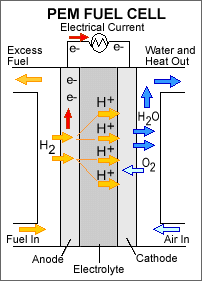
Example of a PEM (proton exchange membrane) fuel cell
The corresponding reactions are:
Oxidation: H2 -> 2H+ + 2e-
Reduction: O2 ->2O2- + 4e-
On the left-hand side, hydrogen (H2) is inserted, where it will be oxidized catalytically and these new hydrogen cations (or protons) pass a n electrolyte. On the right-hand side, air, O2, is inserted, where it will react with the protons (H+) and form water and release heat.
This is quite good for global warming as PEM fuel cells do not produce any carbon dioxide (CO2) (other batteries do produce CO2).