Dealing with Fallout or Detonation Irradiation
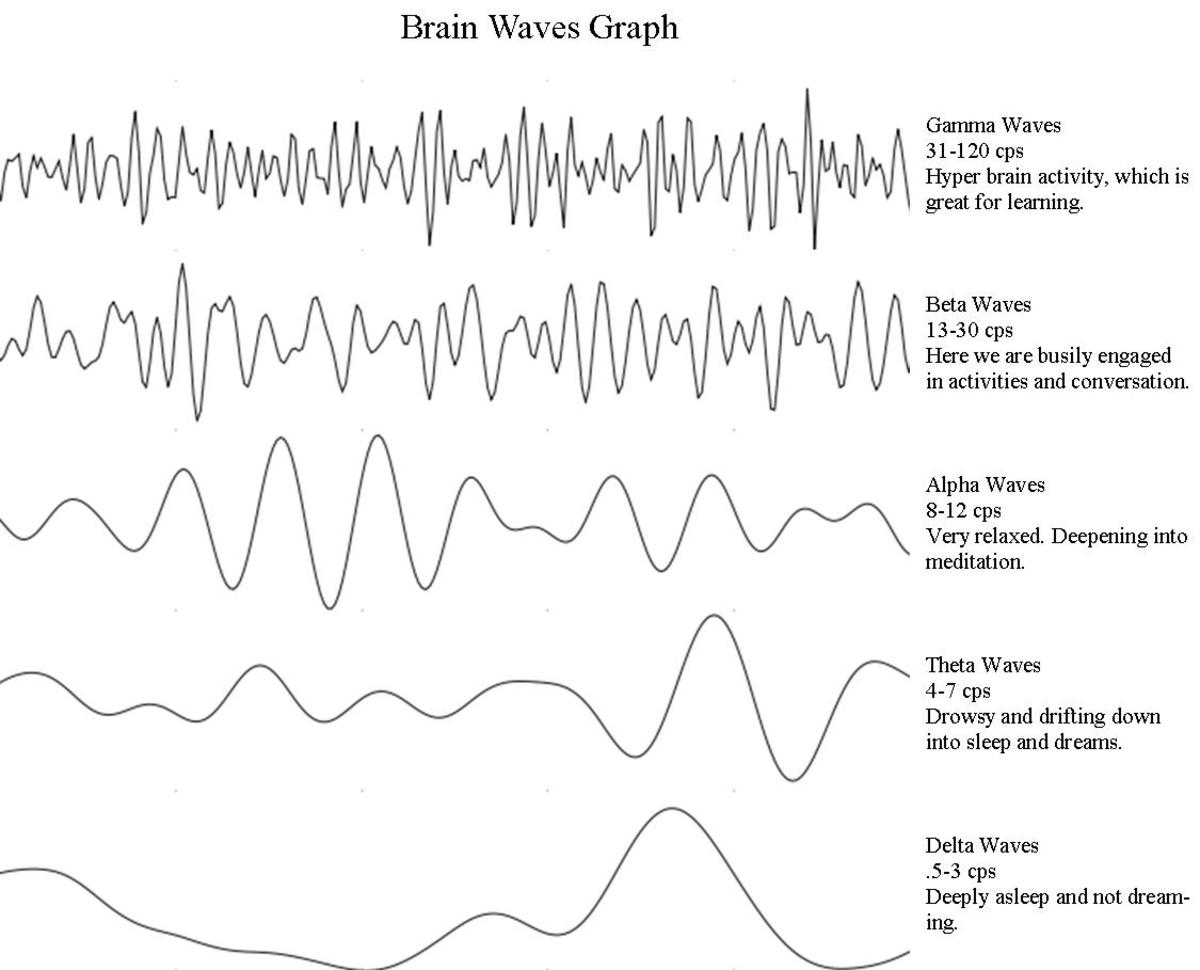
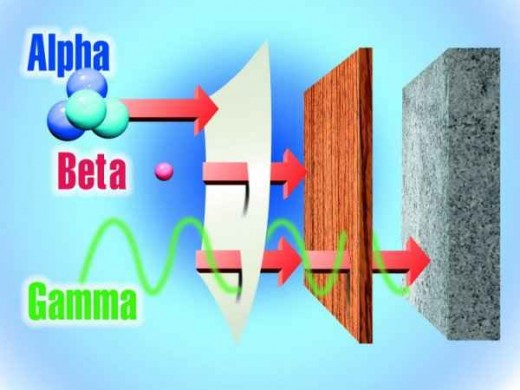
A Pictorial Representation of Various Types of Radiation
The Whys and Hows
In order to effectively deal with radiation, we need to first know what it is and the various ways it works. There are four principle ways that radiation works to disrupt biological systems. To understand how ionization works, one needs to understand the complexities of the atom and the various various elements. To begin, Einstein wrote his seminal paper on the photoelectric effect in 1905 that inadvertently opened the door to quantum mechanics. The photoelectric effect is thus one of the ways that radiation creates damage by breaking down complex organic molecules in your body and releasing free radicals. The free radicals in the body then go on to do further damage, but there are natural ways to deal with this to limit, stop and reverse damage.
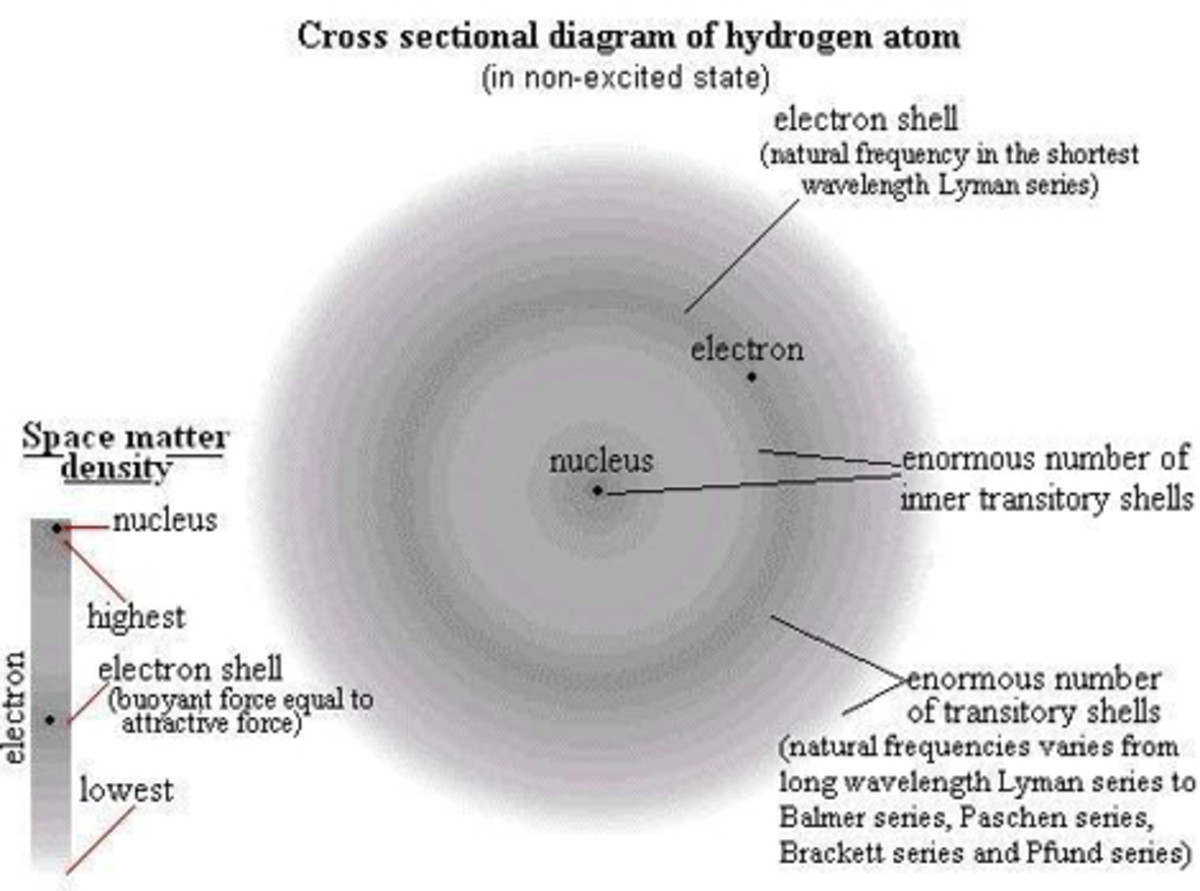
The basic premise of the photoelectric effect is that atomic elements all respond to very specific frequencies in the electromagnetic spectrum. This became the physical explanation for the Fraunhoffer emission-absorption spectra of the elements and this allowed us to define atomic structure. For example, hydrogen has two visible spectral lines, one red and the other blue. There are other lines as well, but they are beyond the visible spectrum. It is interesting to note that photosynthesis works best at the specific hydrogen frequencies where the photoelectric effect exists. When the right frequency of the electromagnetic spectrum interacts with hydrogen, it will ionize, i.e., lose an electron and become an ion, in the case of simple hydrogen, a loose proton. This proton, and also the separated electron are highly reactive and in the right circumstance, are the foundation of chemical energy. In the wrong circumstances, these “loose cannons” can cause a lot of damage. More complex elements have more protons and more electrons and react to different and more frequencies. In order to have a successful ionizing interaction that causes the release of free radicals in the form of electrons or ions, some conditions have to be fulfilled.
-
The exact electromagnetic frequency for the photoelectric effect to occur in any atom must exist.
-
That photon must connect with the electron probability cloud directly. A near miss does not work.
-
Copious amounts of specific frequency photons must be present to increase chances for the photoelectric effect and hence ionization to occur.
-
The atom must be exposed to the radiation.
Human bodies are about 70 percent water by composition. Two out of three atoms in water are hydrogen. Other elements in the body also react to radiation. The more complex the atom, the more frequencies it will respond to. Those who are familiar with radiation technology can design instruments that put off the exact frequencies required in order to effect changes to the target. Some of these changes are necessary in order to explore inside complex systems without dismantling them or to backscatter from the target for other purposes. It takes a lot of radiation in order to create an overall image on film or on a charge coupled device (CCD). Of the two, the CCD is preferred as they are more sensitive, so less radiation is required to activate them. CCDs can be directly coupled with computers and thus will allow real time video imaging such as in backscattering imaging. But real time imaging requires a constant flow of appropriately tuned radiation according to the physics of photoelectrics from specifically tuned emitters. In the case of the human body under various clothing, these have to be tuned to mainly hydrogen and oxygen frequencies as human bodies are mostly water and clothes, unless wet, are not. Dry cloths contain a high percentage of carbon. The tuned radiation passes through the clothes unimpeded and then is backscattered from the surface tissues of the body underneath, reflected back to CCDs. In this case, the frequencies are tuned to those below infra red frequencies and above ultra-violet ones. Why below infra red? A living body emits copious amounts of infra red radiation in an unequal manner. Anything close to the body will absorb this and also emit infra red radiation. The body does not emit any millimetre radiation, nor does it emit ultra violet or soft X-ray. These frequencies are thus useful to penetrate just enough to “strip search” a person without removing clothes, but also “reflect” off the body. The ultra-violet to soft X-rays will penetrate the body to slightly more than an inch which will will involve the endothermis, fat, muscle, cartilage and bones in fingers, capillaries and bodily fluids. This radiation will not penetrate objects like belt buckles, jewelry, plastic containers and hidden munitions and this is just what security is looking for, but violating privacy in the process.
Three other ways that radiation can enter the body are described and involve one form or another of radiological dust.
Alpha Radiation
Alpha radiation is an ejected and ionized helium nucleus. Some effects of alpha radiation as follows: Most alpha radiation is unable to penetrate human skin. Alpha-emitting radiological materials can be harmful to humans if the materials are inhaled, swallowed, or absorbed through open wounds. A variety of instruments has been designed to measure alpha radiation. Specialized training in the use of these instruments is essential for making accurate measurements. A thin-window Geiger-Mueller (GM) probe can detect the presence of alpha radiation. These instruments cannot detect alpha radiation through even a thin layer of water, dust, paper, or other material, because alpha radiation is not penetrating due to its mass and relatively low velocity. Alpha radiation can only travel a short distance (several centimetres) in air and is not an external hazard. Alpha radiation is not able to penetrate clothing. Examples of some alpha radiation emitters are radium, radon, uranium and thorium, just the kinds we would expect as byproducts in the dust from a meltdown or a thermonuclear detonation..
Beta Radiation
Beta radiation is comprised of light, short-range particles and is actually an ejected electron. This can come from the photoelectric effect among other causes. Some characteristics of beta radiation are described as; it may travel several feet in air and is moderately penetrating. Beta radiation is able to penetrate human skin to the "germinal layer," where new skin cells are born. If high levels of beta-emitting contaminants are allowed to remain on the skin for a prolonged period of time, they may cause skin injury and radiation burns similar to a sunburn. Beta emitting contaminants may be harmful if taken internally. Most beta emitters can be detected with a survey instrument and a thin-window GM probe. Some beta emitters, however, produce very low-energy, poorly penetrating radiation that may be difficult or impossible to detect. Examples of these difficult to detect beta emitters are hydrogen3 (tritium), carbon14, and sulphur35. Clothing provides a measure of protection against beta radiation. Some examples of some pure beta emitters are strontium90, carbon14, tritium, and sulphur35. If these get inside the body, they can do some damage over a prolonged time. Strontium90 is absorbed in bones and Carbon14 can wind up anywhere. So can sulphur35. everybody has traces of tritium (heavy water) at all times. Proper nutrition can take care of this kind of trace, but overdoses are much harder to deal with.
Gamma and X Radiation
Gamma radiation and x rays are highly penetrating and extremely energetic electromagnetic radiation. Some characteristics of this type of radiation is as follows. Gamma radiation to soft x-rays are able to travel many feet in air and many inches into human tissue. They readily penetrate most materials including metal and are sometimes called "penetrating" radiation. X rays are like gamma rays, but not as severe. X rays are also penetrating radiation. Sealed radioactive sources and machines that emit gamma radiation and x rays respectively constitute mainly an external hazard to humans. Gamma radiation and x rays are electromagnetic radiation like visible light, radio waves, and ultraviolet light. They can trigger a massive photoelectric effect upon most atoms. These types of electromagnetic radiation differ only in the amount of energy that they have. Gamma rays and hard x rays are the most energetic of these. Dense materials are needed for shielding from gamma radiation such as lead and thick concrete. Clothing provides little effective shielding from penetrating radiation, but can prevent contamination of the skin by gamma-emitting materials should they settle upon you after an atomic blast or a meltdown. Gamma radiation is easily detected by survey meters with a sodium iodide detector probe. Gamma radiation and/or characteristic hard x rays frequently accompany the emission of alpha and beta radiation during radioactive decay that is characteristic of an atomic blast or a nuclear reactor meltdown. Some examples of some gamma radiation emitters are iodine131, cesium137, cobalt60, radium226, and technetium299. All of these are known byproducts of nuclear reactions.
Keeping clean
The first blinding flash of an atomic detonation releases copious amounts of gamma and hard x-ray radiation. Thus at the sight of the blinding flash, it is important to get behind a boulder of thick concrete immediately. The shock wave will follow in short order. In a suitable location, you might even survive the shock wave with an overpressure of 3 or 4 PSI over atmospheric pressure. After the initial blast of an atomic bomb, the radioactive dust begins to settle. A meltdown with an accompanying hydrogen explosion will not produce a blinding flash and the shock wave will be minor but copious amounts of radiological dust will be injected into the atmosphere anyway if Chernobyl and Japan serve as historical reference examples. The worst place is downwind of the initial blast. The dust will contain virtually every known radiological isotope known to man. You need to have a clean set of cloths set aside inside some form of protective shielding. Once the dust has settled, take off your contaminated clothes and put on the clean, dust free clothes. This will eliminate most of the hazards of the settling radiological dust in close proximity to your body. Avoid touching any dust covered surface! Admittedly, you will likely have to walk through radioactive dust, so have a pair of construction rated boots that have steel in them. Do not eat or drink anything with dust in or on it. If prepared well enough, you might even have a micron filtering industrial quality respirator to filter out everything except single ions and electrons. Wear this at all times when outdoors. If radioactive dust is in the air, keep home and office windows, doors and vents closed and sealed. Have fresh air brought in through a filtration system.
Nutrition to Combat Radiological Poisoning
As with many other diseases, radiation that causes creation of free radicals in the body can be dealt with by proper nutrition in a timely way. What is required are foods containing antioxidants, which are capable of neutralizing free radicals by way of stripped electrons and ions in the body. It does not eliminate the source of radiation that is in the body, but antioxidants can help you remain healthy during the period of these isotopes are active in the body. Isotopes have what is called a “half life” of decay, which by definition is the time it takes for half of the total amount of isotope in your body to radiate and become harmless. So if an isotope takes three weeks to reach half life, the radiation level drops by that amount in the same time. After six weeks, the radiation level drops to a quarter and twelve weeks to an eight and so on. Different isotopes have different half lives. For some, the period is weeks and others the time is billions of years. The higher the dose of radiation received, the higher the amount of anti-oxidants is required to deal with the free radicals. The quantity and type of isotopes has to be addressed on an individual basis so treatment for one can be tapered off quickly while another will require life long, low level treatment. For fast decaying isotopes, the large dose is required and matched to the half life, whereas in the slow decay, the homoeopathic approach works best over a lifetime. In addition, certain types of vitamins and minerals are helpful in dealing with radiological poisoning. Getting these in natural form is the best, but failing that, a trusted brand can be useful. Then there are substances that are know to be resilient to ionizing radiation. Among these are items like lotus seeds. They have a natural immunity to radiation caused decay and have been known to sprout after thousands of years lying dormant, even while exposed to ionizing radiation. Taking some of these will transfer the radiation protective alkaloids that your body will require in healing from external and internal radiation exposure. If you suspect radiological poisoning, it is best to get a program going as soon as possible.
As stated, various “common” radiological isotopes have a half life where radiation output decrease by half over certain times. Refer to chart 1 of element-isotopes to see what half life time span exists for these isotopes. As you can see, some decay in seconds while others take billions of years. In a nuclear release from a bomb or a meltdown, almost everything you see in the chart, is released into the air. The more massive stuff will settle out quickly, but unfortunately, the decay is long lasting. The lighter stuff will remain airborne, sometimes for years and much of if has a relatively short half life. Thus it is important to modify your nutrition to meet the challenges presented by radiological hazards in the environment. Recall that radiation intensity varies as the inverse square of the distance from the source. Thus a source that emits 16 rem at 10 feet will fall to 4 rem at 100 feet and so on. Distance from the source is therefore important. This applies to non windy days. For windy conditions, being downwind of a radiological source will skew the intensity to inverse square distance relationship in a lopsided fashion with unpredictable results. Note that in Chart 1 you will see Beta and Alpha decay and no mention of gamma radiation. To emit gamma radiation, an intense reaction is needed that exceeds “critical mass” in order to release gamma radiation. This release comes from the moment of detonation of a nuclear weapon, or at the heart of the meltdown in the line of sight. Beta and Alpha radiation occurs from “passive” isotopes that remain after the detonation cools or the dust is released into the air from a meltdown
The Radiological Isotopes
Isotope
| half-life
| Decay mode
| Periodic symbol
| element number.
|
Caesium-137
| 30.23 years
| Beta -
| Cs
| 55
|
Calcium-37
| 175 milliseconds
| Beta +
| Ca
| 20
|
Carbon-14
| 5730 years
| Beta -
| C
| 6
|
Cobalt-60
| 5.26 years
| Beta -
| Co
| 27
|
Francium-220
| 27.5 seconds
| Alpha
| Fr
| 87
|
Gold-198
| 2.09 days
| Beta -
| Au
| 79
|
Hydrogen-3 (Tritium)
| 12.26 years
| Beta -
| H
| 1
|
Iodine-131
| 8.07 days
| Beta -
| I
| 53
|
Iron-53
| 8.51 minutes
| Beta +
| Fe
| 26
|
Krypton-85
| 10.76 years
| Beta -
| Kr
| 36
|
Neon-19
| 17.2 seconds
| Beta +
| Ne
| 10
|
Nitrogen-16
| 7.2 seconds
| Beta -
| N
| 7
|
Phosphorous-32
| 14.3 days
| Beta -
| P
| 15
|
Plutonium-239
| 2.44X10^4 years
| Alpha
| Pu
| 94
|
Potassium-37
| 1.23 seconds
| Beta +
| K
| 19
|
Potassium-42
| 12.4 hours
| Beta -
| K
| 19
|
Radium-236
| 1000 years
| Alpha
| Ra
| 88
|
Radon-222
| 3.52 days
| Alpha
| Rn
| 86
|
Strontium-90
| 28.1 years
| Beta -
| Sr
| 38
|
Technetium-99
| 2.13X10^5 years
| Beta -
| Te
| 52
|
Thorium-232
| 1.4X10^10 years
| Alpha
| Th
| 90
|
Uranium-233
| 1.62X10^5 years
| Alpha
| U
| 92
|
Uranium-235
| 7.1X10^8 years
| Alpha
| U
| 92
|
Uranium-238
| 4.51X10^9 years
| Alpha
| U
| 92
|
Chart 1 - The atomic "element" number tells us how many protons are in the nucleus whereas the number at the end of the name tells us how many sub-atomic particles are in the nucleus. The number of neutrons is the nucleon number minus the proton numb
We now look at radiation measurement. Like everything else, we have ways to measure quantities if radiation, which is accomplished with a Geiger counter or a personal dosimeter. There are a number of systems in place to measure radiation, but for our purposes, we will deal in millirem and Rem measures. Consulting Chart 2, we find what an average person is likely to be exposed to in a non contaminated setting on an annual basis. With radiological contamination, these figures then become the base and anything delivered by fallout is in addition to this.
External Background Radiation from sun, cosmos
| 60 millirem/yr, US Average
|
Natural Potassium-40 and Other Body Radioactivity
| 40 millirem/yr
|
Air Travel Round Trip (NY and LA or equivalent)
| 5 millirem
|
Chest X-Ray Effective Dose (average three shots)
| 10 millirem per film taken
|
Radon in the Home from ground level
| 200 millirem/yr (variable)
|
Man-Made radiation (medical x rays, etc.)
| 60 millrem/yr (average)
|
Chart 2
While looking at Chart 2, consider that an “acceptable” amount of radiation per year according to the American Medical Association (AMA) is 5,000 millirem or 5 rem at the maximum. According to the above, the average person gets anywhere from 300 to 395 millirem a year. This suggests that in nature, we get 300 miilrem a year. Anything beyond this wanders increasingly into dangerous and unknown territory. Radiation comes in many forms as we know, but there are four principle radiation types; being alpha, beta, gamma and X-radiation. Consider the fact that medical or dental X-rays are usually taken over limited areas of the body as opposed to the entire body. Using mathematics and the known quantity of radiation from a source, we can calculate what a whole isotope spectrum of fallout from meltdown or atomic detonation exposes the entire body to in addition to the base amount. We also know what occurs in cancer therapy where radiological sources such as cobalt-60 are used to kill cancer. Typically, these patients will lose hair demonstrating that they are receiving dangerous levels of ionizing radiation. What we don't know is the radiative output of meltdowns and atomic detonations until a dosimeter or a Geiger counter is read. We can surmise for the most part that exposure in most cases is somewhat less than a standard chest X-ray, but over the entire body from all directions instead of the typical two or three X-rays for a chest examination in specific directions.
The radiation that we are concerned about in this application is electromagnetic radiation from gamma and x-ray sources that triggers the photoelectric effect. It is the photoelectric effect in these applications that causes ionization in the body, producing copious amounts of free radicals that leads to that tired feeling. There is confusion over radiation measure as well, due to the existence of several scales. But these are cross convertible and we can still come to a sense of dosage.
Once exposed to a large quantity and spectrum of radiological isotopes, the damage has been done, which some think can lead to cancers like skin melanomas and leukemia. It is unknown what the Rem or Rontgen dosage is that a person receives from one of these sources for reasons mentioned above, but there is good knowledge of what is an acceptable dosage for a person to “safely” receive on an annual basis externally and/or internally. Nuclear medicine from experience sets the standard at 5,000 millirems (5 Rem) per year for the entire body. No two organ systems are the same for radiation susceptibility. Some organ like the intestines are more easily harmed than skeletal articulating muscles. Organs like eyes are very delicate and almost entirely within the range of such massive exposure. As some radiation penetrates up to three centimeters into soft tissue, organs like fingers, noses, toes, penises and testicles have radiation go right through them with little backscatter. This means that radiation passes right through the finer points of the body, but does ionizing damage all the way through in the process; hence the possibility of temporary or permanent sterilization of males due to complete passage of ionizing radiation through their testicles. Even if full recovery is made from sterilization, the chance for mutations in future generations is increased numerically by the number millirem received. As a gauge, frequent flying males have a greater chance of complete sterilization or if they recover, greater chances of contributing to mutations in future generations. The recent use of imaging backscattering scanners at airports and on the road give us more radiation. It is interesting to note that security personnel who use these devices must remain at least 50 feet away as they will be running these devices on jet passengers thousands of times a day. Given the radiation intensity inverse distance square relation, we can gain a sense of proximity hazard to such radiological instruments. The closer one is to the radiation source, the greater the exposure to ionizing radiation, hence the required standing off by security when the machine is fired up on passenger after passenger. X-ray technicians are also required to stand off and often do this from a sealed closet near the x-ray machine.
What you may feel after being irradiated is a feeling of unease and slight nausea. You may also feel tired as if you just had an intense work out. It may persist for some time; in some cases for a few days. There may difficulty in falling to sleep and odd pains. Your pulse will seem to race and be heavy which is typical of some shock and trauma to the body. There might even be a temporary loss of appetite. To simplify, if this is the case, you have a light case of radiation poisoning. If not treated, you will have lowered immunity for about a year and be susceptible to getting ill easily. But, you can take immediate measures to counteract these tenancies. To counteract this immediately, you need antioxidants to mop up free radicals such as strong green tea for drinking periodically and 400 IU vitamin E. To restore appetite, you will need medical approved and legally sanctioned marijuana. For longer term protection against massive DNA damage, you will need to procure and eat lotus seeds and other herbs that will counteract the long term effects of ionizing radiation. For iodine, you need to procure and eat kelp, dulse or nori such as used for making sushi. If you live near a nuclear power plant that has gone into meltdown, you will need to make these items part of you daily diet as well as taking all other precautions.
Are you uncertain as to how much exposure you've had? There is a home grown way to estimate the amount of radiation you have absorbed by the rapid onset of radiological based symptoms. This can serve as a kind of rough gauge of what you need to do and how long. 5 Rem of radiation is considered to not cause symptomatic response and that is what is considered a total safe does for a year. At 20 to 50 Rem, there is a fall off of white blood cells counts and lessened immunity that can lead to easily getting sick and frequent illness, but there are few immediate symptoms. The symptoms will show after and you will catch “every bug” out there until the body reasserts autoimmune function to the full. This may take up to a year. Above 50 Rem gives one mild radiation sickness that is described as a feeling of nausea extreme tiredness. If you have the symptoms described, then you have received ionizing radiation to the tune of 50 to 100 Rem; up to 20 times the annual allowance. This is an important benchmark, so take note, even keeping a journal if you are a frequent flier or have been exposed to a meltdown or an atomic blast. At 100 to 200 Rem, there are symptoms that increase with dosage with a 10 percent chance of fatality in 30 days after exposure at the high end of dosage. Dosages up to 300 Rem cause moderate symptoms with hair loss, vomiting and diarrhea at the high end with a 35 percent chance of death in 30 days. Up to 400 Rem, these symptoms become severe and include bleeding from the mouth and bowels and a 50 percent chance of death after 30 days. Anything beyond this is usually considered fatal either shortly after or a couple of months depending on doses above 400 Rem. These statistics have come to us via atom bomb experiments, nuclear war and nuclear medicine. Now we have a new concern in the form of nuclear reactor meltdowns due to natural disasters like earthquakes and tsunamis. Many nuclear power generating plants are located near coast lines for easy access to cooling water. Many are also built on fault lines and are susceptible to being disabled during an earthquake. To top it all off, war plans target nuclear power plants as top priority targets for obvious reasons if you have followed this so far.
In conclusion, there is available from a wide variety of sources, guides as to what nutrients are available in which foods that will assist you in protection and healing. You will need to get these foods as fresh as possible and avoid overcooking them.
Atomic detonation: Watch out for the post explosion dust
The meltdown scenarioL Watch out for the dust!
References
One mineral not listed is iodine that everyone is concerned about and potassium iodine is being recommended. But natural sources of iodine exist in seaweeds such as kelp, dulse and nori. You should include these in your diet for optimizing nutrition and immunity.
http://ublib.buffalo.edu/libraries/projects/cases/amazon.html
http://www.astrobio.net/pressrelease/2598/making-artificial-bacteria
http://www.theregister.co.uk/2007/10/08/venter_synthetic_chromosome/
http://www.americanhospice.org/index.php?option=com_content&task=view&id=48&Itemid=8
Understanding Uranium and its Role in Radiation
- Uranium: It's Creation, Use and Misuse
This is the seaward side of Ballentyne peer where the nuclear accident ship is located. This particular ship is not the one in question. Beyond the peer is a park and the Downtown East Side IDTES), one of...